Tuning the Nucleophilicity of Anion in Lithium Salt to Enable an Anion-Rich Solvation Sheath for Stable Lithium Metal Batteries
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Traditional lithium salts typically adhere to the designing principles of enhancing cation-anion dissociation degree to obtain a high electrolyte conductivity. This promotes the invention of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), where the symmetric electron-withdrawing trifluoromethanesulfonyl groups significantly delocalize the negative charge density around the nitrogen atom, thereby weakening the electrostatic interaction between Li+ and the anion. Herein, deviating from the general principle, lithium (methanesulfonyl)(trifluoromethanesulfonyl) imide (LiMTFSI) is deliberately designed by substituting a unilateral electron-withdrawing trifluoromethyl (─CF3) group of LiTFSI with an electron-donating methyl (─CH3) group, to tune the nucleophilicity of the anion. This modification enhances Li-anion interaction, causing the anion to replace the solvent molecules in the Li+ solvation shell. Additionally, the MTFSI− anion exhibits an elevated donor number to facilitate the solubility of LiNO3 in carbonate-based electrolytes. The synergistic effect of these changes suppresses the decomposition of solvent and helps construct a stable solid electrolyte interphase (SEI) enriched with multiple inorganic lithium salts (e.g., Li2S, Li3N, and LiNxOy) on the Li metal anode, which enables the 500 mAh Li||LiNi0.5Co0.2Mn0.3O2 pouch cell to operate steadily for 150 cycles. It is believed this work would provide new insights and another dimension for designing functional anions beyond their role as charge carriers.
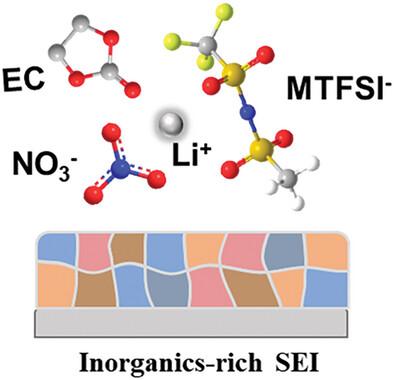
调整锂盐中阴离子的亲核性,为稳定的金属锂电池提供富含阴离子的溶解鞘
传统的锂盐通常遵循提高阳离子-阴离子离解度以获得高电解质电导率的设计原则。双(三氟甲烷磺酰基)亚胺锂(LiTFSI)的对称性夺电子三氟甲烷磺酰基使氮原子周围的负电荷密度显著分散,从而削弱了 Li+ 与阴离子之间的静电作用。在此,我们偏离一般原理,特意设计了(甲磺酰基)(三氟甲磺酰基)亚胺锂(LiMTFSI),用一个给电子的甲基(-CH3)基团取代 LiTFSI 的单侧抽电子的三氟甲基(-CF3)基团,以调节阴离子的亲核性。这种改性增强了锂阴离子的相互作用,使阴离子取代了 Li+ 溶壳中的溶剂分子。此外,MTFSI- 阴离子的供体数增加,从而提高了 LiNO3 在碳酸盐电解质中的溶解度。这些变化的协同效应抑制了溶剂的分解,有助于在锂金属阳极上构建富含多种无机锂盐(如 Li2S、Li3N 和 LiNxOy)的稳定固态电解质相(SEI),从而使 500 mAh Li||LiNi0.5Co0.2Mn0.3O2 袋式电池能稳定运行 150 个循环。相信这项工作将为设计功能阴离子提供新的见解和另一个维度,而不仅仅是其作为电荷载体的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


