Investigation of the Effect of the Third Cation M (M = Mg, Al, Mn, and Fe) on the Properties and Catalytic Behavior in Ethane Oxidative Dehydrogenation of M-NiNbO Mixed Oxides
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
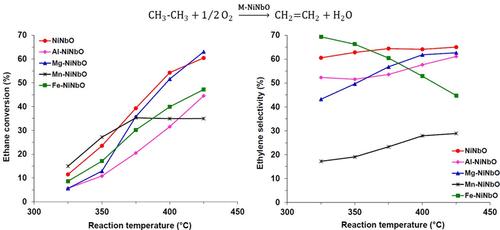
This work examines the effect of incorporating a third cation M (M = Mg, Al, Mn, or Fe) into Nb-promoted NiO catalysts (Ni0.85Nb0.15O) on their physicochemical properties and catalytic performance in the oxidative dehydrogenation (ODH) of ethane into ethylene. Therefore, a series of mixed oxides with the composition Ni0.765Nb0.135M0.1O was synthesized, characterized, and analyzed for catalytic behavior. The addition of the third cation markedly modified the redox and semiconductive properties of the catalysts, thus affecting their ODH performance. The selectivity toward ethylene was strongly dependent on the cation modifier, as it modulated the density of nonselective sites on the catalyst surface. In the low-temperature region, Mn-NiNbO demonstrated the highest conversion rates but with the lowest ethylene selectivity, whereas Fe-NiNbO, despite being less active than the undoped NiNbO, exhibited the highest ethylene selectivity. Overall, undoped NiNbO emerged as the best catalyst in terms of both activity and selectivity at isoconversion. All catalysts underwent partial reduction under reaction conditions, with the degree of reduction inversely correlated with the specific reaction rates. Although none of the catalysts remained stable at 400 °C, Al-NiNbO showed the least deactivation over time. The deactivation was linked to a reduction in p-type conductivity and a loss of redox functionality during operation.
研究第三阳离子 M(M = Mg、Al、Mn 和 Fe)对 M-NiNbO 混合氧化物乙烷氧化脱氢的性质和催化行为的影响
本研究探讨了在铌促进的氧化镍催化剂(Ni0.85Nb0.15O)中加入第三阳离子 M(M = Mg、Al、Mn 或 Fe)对其理化性质以及在乙烷氧化脱氢 (ODH) 成乙烯过程中催化性能的影响。因此,我们合成了一系列成分为 Ni0.765Nb0.135M0.1O 的混合氧化物,并对其催化行为进行了表征和分析。第三种阳离子的加入明显改变了催化剂的氧化还原性和半导电性,从而影响了它们的 ODH 性能。对乙烯的选择性在很大程度上取决于阳离子改性剂,因为它调节了催化剂表面非选择性位点的密度。在低温区域,Mn-NiNbO 的转化率最高,但乙烯选择性最低,而 Fe-NiNbO 虽然活性低于未掺杂的 NiNbO,但乙烯选择性最高。总体而言,未掺杂的镍铌氧化物在异构转化的活性和选择性方面都是最好的催化剂。在反应条件下,所有催化剂都会发生部分还原,还原程度与特定反应速率成反比。虽然没有一种催化剂能在 400 °C 下保持稳定,但 Al-NiNbO 的失活程度随时间的推移最小。失活与 p 型电导率的降低和运行过程中氧化还原功能的丧失有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


