Reprogramming astroglia into neurons with hallmarks of fast-spiking parvalbumin-positive interneurons by phospho-site–deficient Ascl1
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Cellular reprogramming of mammalian glia to an induced neuronal fate holds the potential for restoring diseased brain circuits. While the proneural factor achaete-scute complex-like 1 (Ascl1) is widely used for neuronal reprogramming, in the early postnatal mouse cortex, Ascl1 fails to induce the glia-to-neuron conversion, instead promoting the proliferation of oligodendrocyte progenitor cells (OPC). Since Ascl1 activity is posttranslationally regulated, here, we investigated the consequences of mutating six serine phospho-acceptor sites to alanine (Ascl1SA6) on lineage reprogramming in vivo. Ascl1SA6 exhibited increased neurogenic activity in the glia of the early postnatal mouse cortex, an effect enhanced by coexpression of B cell lymphoma 2 (Bcl2). Genetic fate-mapping revealed that most induced neurons originated from astrocytes, while only a few derived from OPCs. Many Ascl1SA6/Bcl2-induced neurons expressed parvalbumin and were capable of high-frequency action potential firing. Our study demonstrates the authentic conversion of astroglia into neurons featuring subclass hallmarks of cortical interneurons, advancing our scope of engineering neuronal fates in the brain.
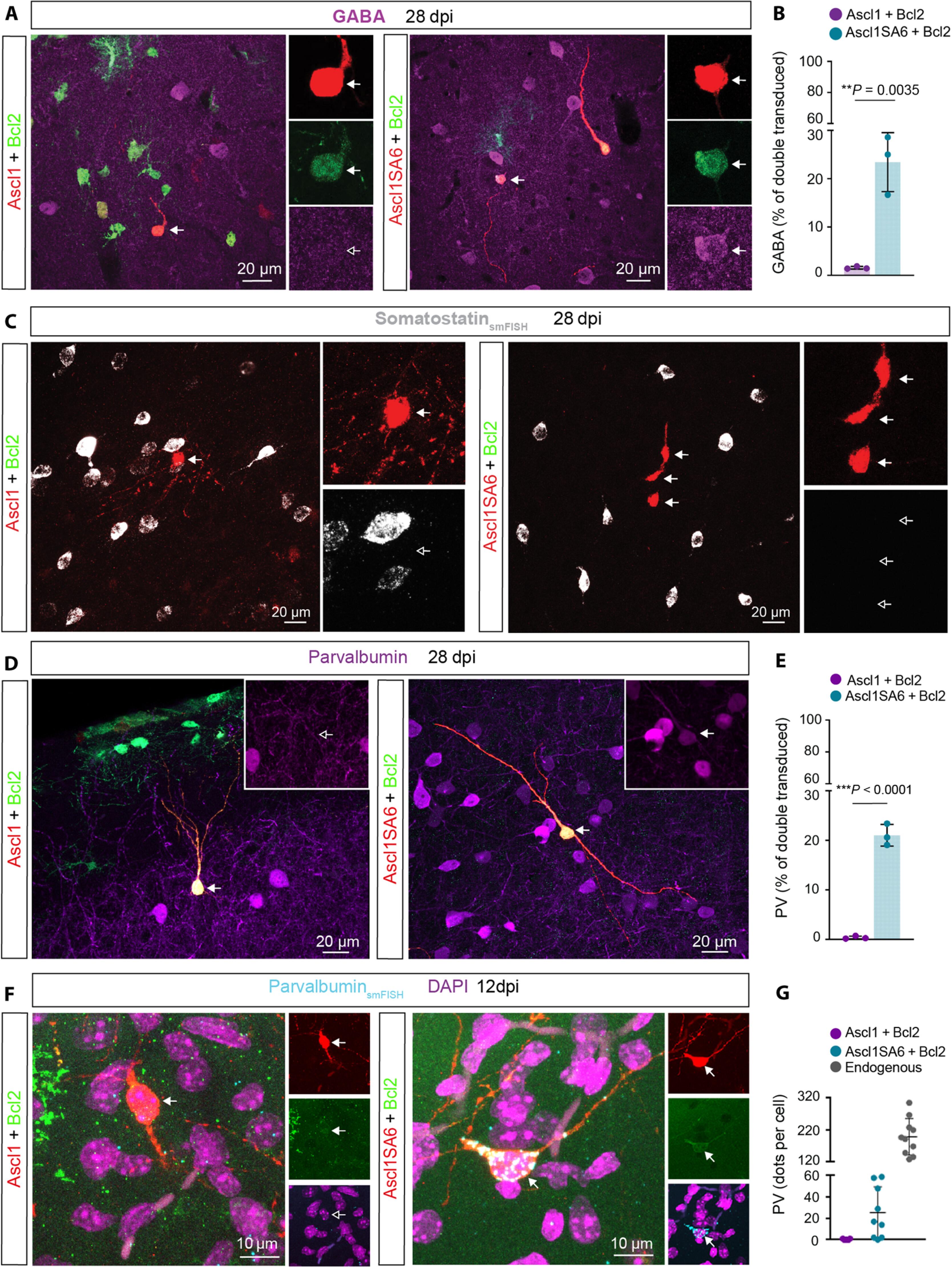
通过磷酸化位点缺陷Ascl1将星形胶质细胞重编程为具有快速尖峰副发光素阳性中间神经元特征的神经元
将哺乳动物神经胶质细胞重编程为诱导神经元命运有可能恢复患病的大脑回路。尽管朊病毒因子Achaete-scute complex-like 1(Ascl1)被广泛用于神经元重编程,但在小鼠出生后早期皮层中,Ascl1不能诱导胶质细胞向神经元的转化,反而促进了少突胶质细胞祖细胞(OPC)的增殖。由于Ascl1的活性受翻译后调控,我们在此研究了将六个丝氨酸磷酸化受体位点突变为丙氨酸(Ascl1SA6)对体内谱系重编程的影响。Ascl1SA6在出生后早期小鼠皮层神经胶质中表现出更强的神经源活性,B细胞淋巴瘤2(Bcl2)的共表达增强了这种效应。基因命运图谱显示,大多数诱导的神经元起源于星形胶质细胞,只有少数神经元来源于OPCs。许多Ascl1SA6/Bcl2诱导的神经元表达副白蛋白,并能进行高频率的动作电位发射。我们的研究表明,星形胶质细胞能真实地转化为具有皮质中间神经元亚类特征的神经元,从而推进了我们的大脑神经元命运工程的范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


