Accelerated Alzheimer’s Aβ-42 secondary nucleation chronologically visualized on fibril surfaces
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Protein fibril surfaces tend to generate toxic oligomers catalytically. To date, efforts to study the accelerated aggregation steps involved with Alzheimer’s disease–linked amyloid-β (Aβ)–42 proteins on fibril surfaces have mainly relied on fluorophore-based analytics. Here, we visualize rare secondary nucleation events on the surface of Aβ-42 fibrils from embryonic to endpoint stages using liquid-based atomic force microscopy. Nanoscale imaging supported by atomic-scale molecular simulations tracked the adsorption and proliferation of oligomeric assemblies at nonperiodically spaced catalytic sites on the fibril surface. Upon confirming that fibril edges are preferential binding sites for oligomers during embryonic stages, the secondary fibrillar size changes were quantified during the growth stages. Notably, a small population of fibrils that displayed higher surface catalytic activity was identified as superspreaders. Profiling secondary fibrils during endpoint stages revealed a nearly threefold increase in their surface corrugation, a parameter we exploit to classify fibril subpopulations.
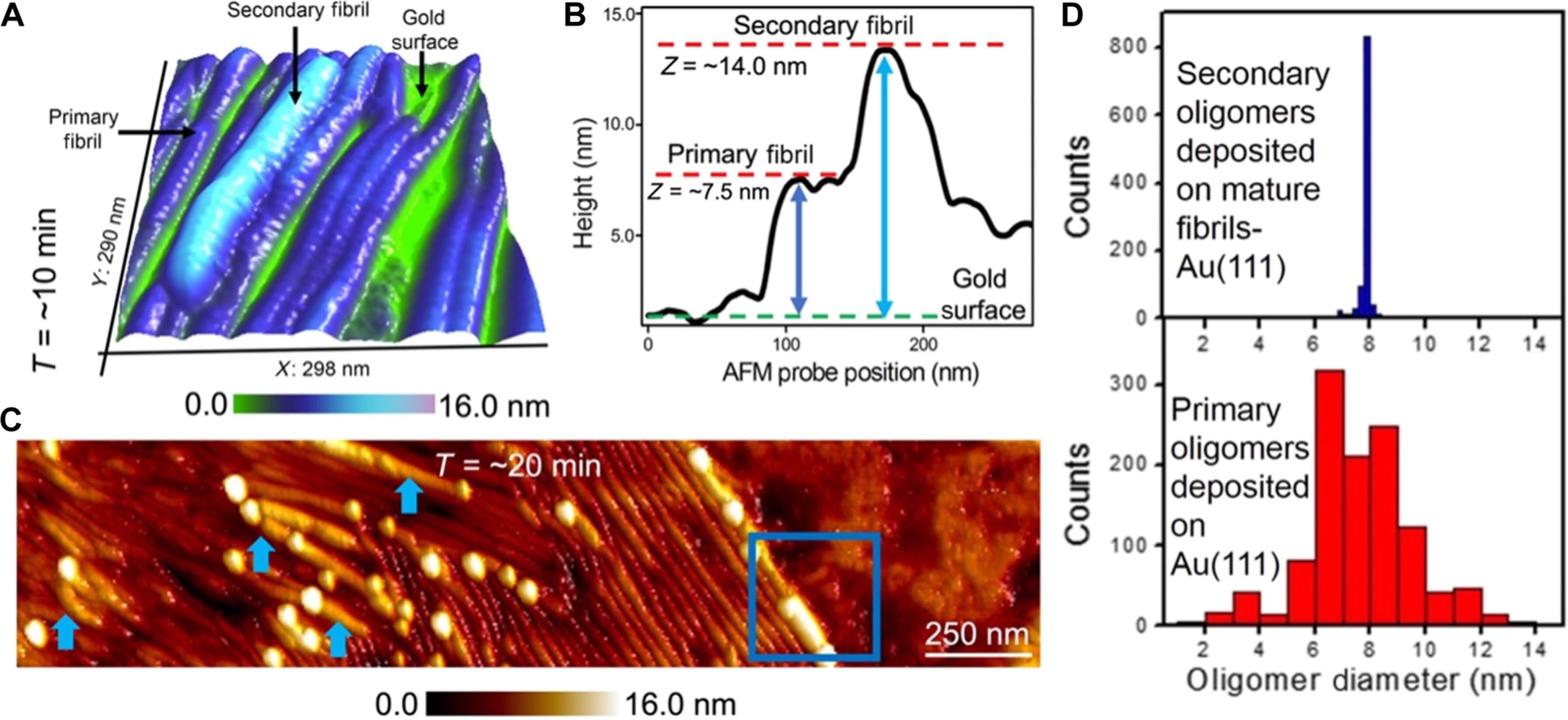
从纤维表面可视化角度加速阿尔茨海默氏症 Aβ-42 二次成核的时间进程
蛋白质纤维表面往往会催化产生有毒的低聚物。迄今为止,研究与阿尔茨海默病相关的淀粉样蛋白-β(Aβ)-42 蛋白在纤维表面的加速聚集步骤主要依赖于基于荧光团的分析方法。在这里,我们利用液基原子力显微镜观察了 Aβ-42 纤维表面从胚胎到终点阶段的罕见二次成核事件。在原子尺度分子模拟的支持下,纳米级成像追踪了纤维表面非周期性间隔催化位点上低聚物集合体的吸附和增殖。在确认纤维边缘是胚胎阶段低聚物的优先结合点后,对生长阶段的次生纤维大小变化进行了量化。值得注意的是,一小部分显示出较高表面催化活性的纤维被鉴定为超级扩散体。在终点阶段对次生纤维进行的分析表明,它们的表面波纹增加了近三倍,我们利用这一参数对纤维亚群进行了分类。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


