Late acquisition of erect hindlimb posture and function in the forerunners of therian mammals
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The evolutionary transition from early synapsids to therian mammals involved profound reorganization in locomotor anatomy and function, centered around a shift from “sprawled” to “erect” limb postures. When and how this functional shift was accomplished has remained difficult to decipher from the fossil record alone. Through biomechanical modeling of hindlimb force-generating performance in eight exemplar fossil synapsids, we demonstrate that the erect locomotor regime typifying modern therians did not evolve until just before crown Theria. Modeling also identifies a transient phase of increased performance in therapsids and early cynodonts, before crown mammals. Further, quantifying the global actions of major hip muscle groups indicates a protracted juxtaposition of functional redeployment and conservatism, highlighting the intricate interplay between anatomical reorganization and function across postural transitions. We infer a complex history of synapsid locomotor evolution and suggest that major evolutionary transitions between contrasting locomotor behaviors may follow highly nonlinear trajectories.
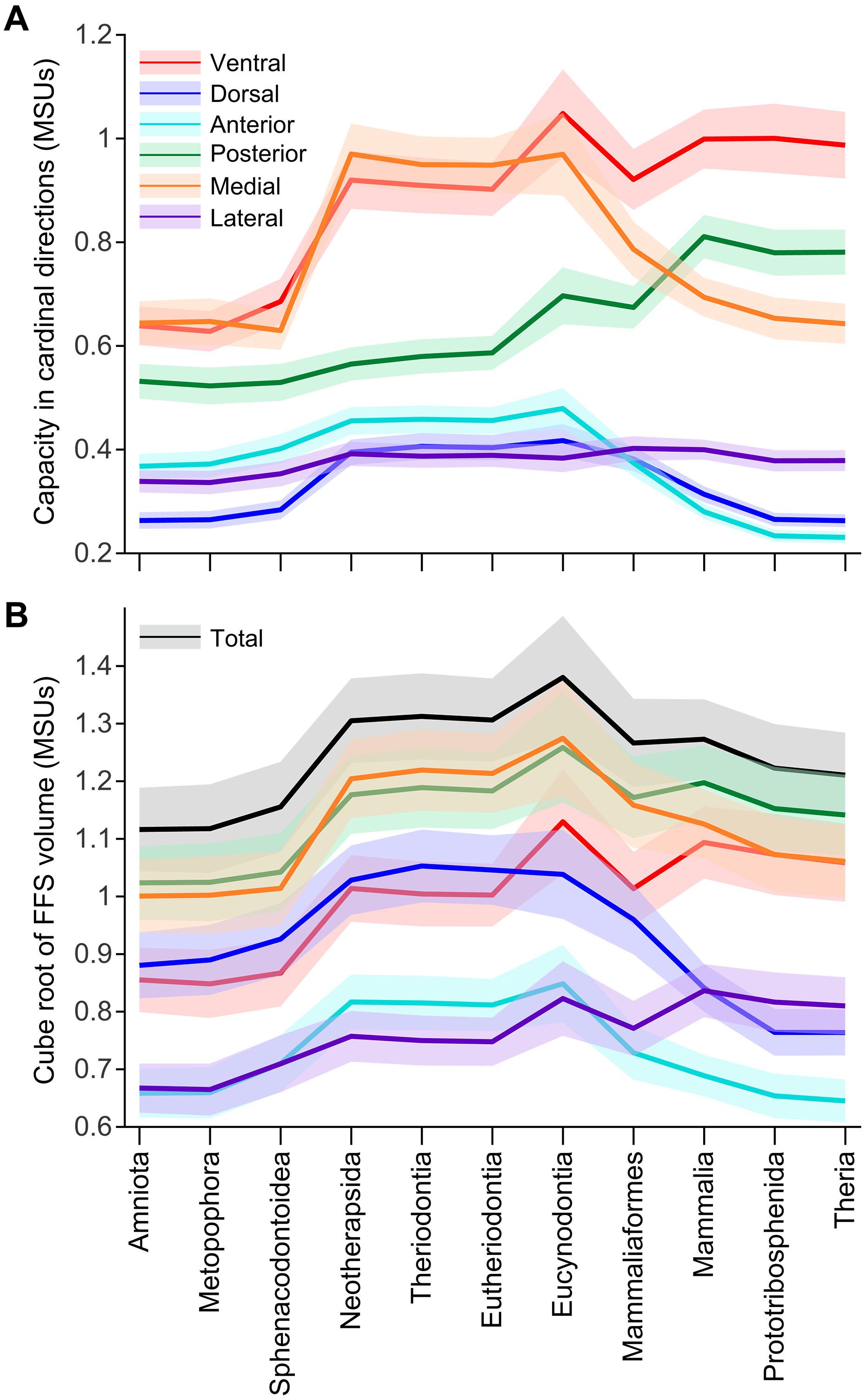
后肢直立姿势和功能在有兽类哺乳动物前身的晚期获得
从早期合趾类哺乳动物到有趾类哺乳动物的进化转变涉及运动解剖学和功能的深刻重组,其核心是从 "匍匐 "到 "直立 "的肢体姿势转变。仅从化石记录来看,这种功能转变是何时以及如何完成的,一直难以破解。通过对八种典型合趾类化石的后肢发力性能进行生物力学建模,我们证明了现代食蚁兽典型的直立运动机制直到冠突伪尾柱虫之前才演化出来。建模还发现,在冠哺乳动物之前,有蹄类和早期犬齿类动物的运动性能曾有过短暂的提高。此外,对主要臀部肌肉群的整体行动进行量化表明,功能的重新部署与保守并存,突出了姿势转换过程中解剖重组与功能之间错综复杂的相互作用。我们推断了滑翔类动物运动进化的复杂历史,并认为对比强烈的运动行为之间的主要进化转变可能遵循高度非线性的轨迹。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


