Development of an Intravenously Stable Disulfide-Rich Peptide for the Treatment of Chemotherapy-Induced Neuropathic Pain
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
α-conotoxins (α-Ctxs), a class of disulfide-rich conopetides, are excellent drug leads due to their small size, high selectivity, and potency for specific membrane receptors and ion channels involved in pain transmission. However, their high susceptibility to proteolytic degradation limits their therapeutic potential. In this study, we designed and synthesized a series of conformationally stable analogues of α-Ctx Mr1.1[S4Dap] using various structural optimization strategies. The Mr1.1[S4Dap, C16Pen] analogue maintained potency at human α9α10 nicotinic acetylcholine receptors, with a half-maximal inhibitory concentration (IC50) of 4 nM. It exhibited over a 5-fold increase in serum stability compared to Mr1.1[S4Dap], without disrupting its overall conformation. Furthermore, intravenous application of Mr1.1[S4Dap, C16Pen] showed potent analgesic activity in oxaliplatin-induced cold allodynia, indicating a high potential for drug development. Overall, the results from this study provide valuable insights for optimizing the serum stability of disulfide-rich peptides in future therapeutic applications.
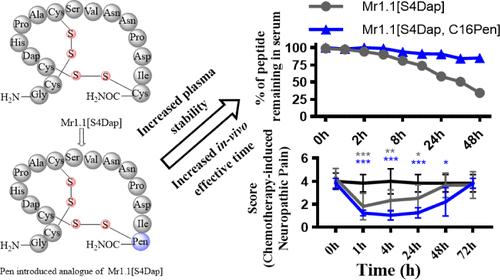
开发用于治疗化疗引起的神经病理性疼痛的静脉注射稳定富二硫化物肽
α-芋螺毒素(α-Ctxs)是一类富含二硫化物的锥形酮类化合物,由于其体积小、选择性高、对参与痛觉传递的特定膜受体和离子通道具有强效作用,因此是极好的药物先导。然而,它们极易被蛋白水解,这限制了它们的治疗潜力。在本研究中,我们采用各种结构优化策略设计并合成了一系列构象稳定的 α-Ctx Mr1.1[S4Dap] 类似物。Mr1.1[S4Dap, C16Pen]类似物对人类α9α10烟碱乙酰胆碱受体保持了效力,半最大抑制浓度(IC50)为 4 nM。与 Mr1.1[S4Dap]相比,它的血清稳定性提高了 5 倍以上,而且不会破坏其整体构象。此外,Mr1.1[S4Dap, C16Pen]的静脉注射对奥沙利铂诱导的冷异感显示出了强大的镇痛活性,这表明它具有很大的药物开发潜力。总之,这项研究的结果为优化富二硫化物多肽在未来治疗应用中的血清稳定性提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


