Light-Controlled Anticancer Activity and Cellular Uptake of a Photoswitchable Cisplatin Analogue
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
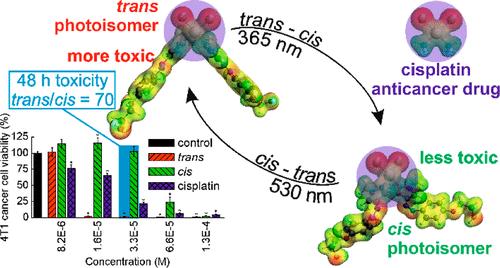
A photoactive analogue of cisplatin was synthesized with two arylazopyrazole ligands, able to undergo trans–cis/cis–trans photoisomerizations. The cis photoisomer showed a dark half-life of 9 days. The cytotoxicities of both photoisomers of the complex were determined in several cancer and normal cell lines and compared to that of cisplatin. The trans photoisomer of the complex was much more cytotoxic than both the cis photoisomer and cisplatin, and was more toxic for cancer (4T1) than for normal (NMuMG) murine breast cells. 4T1 cell death occurred through necrosis. Photoisomerization of the trans and cis photoisomers internalized by the 4T1 cells increased and decreased their viability, respectively. The cellular uptake of the trans photoisomer was stronger than that of both the cis photoisomer and cisplatin. Both photoisomers interacted with DNA faster than cisplatin. The trans photoisomer was bound stronger by bovine serum albumin and induced a greater decrease in cellular glutathione levels than the cis photoisomer.
光控型顺铂类似物的抗癌活性和细胞吸收
我们用两个芳基氮吡唑配体合成了一种顺铂的光活性类似物,这种类似物能够进行反式-顺式/顺式-反式光异构化。顺式光异构体的黑暗半衰期为 9 天。在几种癌症和正常细胞系中测定了复合物的两种光异构体的细胞毒性,并与顺铂的细胞毒性进行了比较。复合物的反式光异构体比顺式光异构体和顺铂都具有更强的细胞毒性,对癌症细胞(4T1)的毒性比对正常细胞(NMuMG)的毒性更强。4T1 细胞通过坏死死亡。4T1 细胞内含的反式和顺式光异构体的光异构化分别增加和降低了它们的存活率。细胞对反式光异构体的吸收强于顺式光异构体和顺铂。两种光异构体与 DNA 的相互作用都比顺铂快。反式光异构体与牛血清白蛋白的结合力更强,与顺式光异构体相比,反式光异构体引起的细胞谷胱甘肽水平下降幅度更大。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


