Generation of alkene radical cation for thioxanthone-TfOH complex-catalyzed intramolecular cyclization using a photoredox catalysis strategy
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
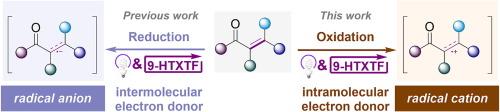
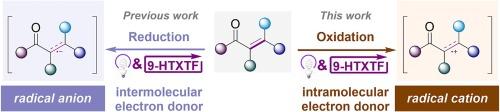
Photo catalysis has comprehensively become a powerful tool in organic synthesis, and organic molecules are thriving as catalyst. The thioxanthone-TfOH complex (9-HTXTF) as photoredox catalyst with high oxidative capacity can be applied in single electron reduction of alkene affording alkene radical anion as a key intermediate. To transform this intermediate from radical anion to radical cation, a well-designed strategy is proposed with N-arylacrylamides as substrate. Based on its single electron transfer (SET) with 9-HTXTF*, N-radical cation is generated and then transformed to alkene radical cation by intramolecular conjugated system. By using this photoredox catalysis strategy, we developed a 9-HTXTF-catalyzed photochemical cyclization of alkenes, which further expands the applications of this catalyst. The entire cyclization is metal-free and without sacrificing agents, which conforms to atom economy and environmental friendliness.
利用光氧化催化策略生成硫黄酮-TfOH 复合物催化分子内环化的烯自由基阳离子
光催化已全面成为有机合成的有力工具,而有机分子作为催化剂的作用也在蓬勃发展。作为具有高氧化能力的光氧化催化剂,硫氧杂环酮-TfOH 复合物(9-HTXTF)可用于烯的单电子还原反应,生成烯自由基阴离子作为关键中间体。为了将该中间体从自由基阴离子转化为自由基阳离子,我们提出了一种以 N-芳基丙烯酰胺为底物的精心设计的策略。根据其与 9-HTXTF* 的单电子转移(SET),生成 N-自由基阳离子,然后通过分子内共轭体系转化为烯自由基阳离子。利用这种光氧化催化策略,我们开发出了 9-HTXTF 催化的烯烃光化学环化反应,进一步拓展了该催化剂的应用领域。整个环化过程不需要金属,也不需要牺牲药剂,符合原子经济性和环境友好性的要求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


