High-Molecular-Weight Biobased Polycarbonate Preparation Using Metal-Free Deep Eutectic Solvents: Efficient Activation of the Hydroxyl Groups
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
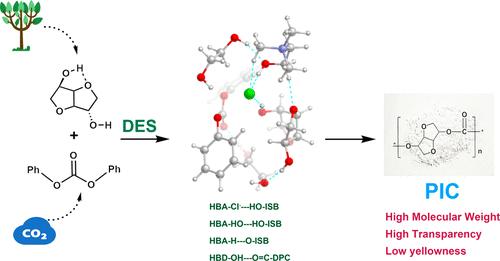
Activating hydroxyl groups in monomers is crucial for preparing high-molecular-weight bioderived polycarbonates. Herein, we developed metal-free deep eutectic solvents (DESs) to activate endo- and exo-hydroxyl groups in isosorbide (ISB) through hydrogen bonding interactions, facilitating the preparation of poly(isosorbide carbonate) (PIC) by melt polycondensation with diphenyl carbonate (DPC). Catalytic evaluation results indicated that the Brønsted acid–base properties of DESs had a greater impact on the Mn of PIC than the molecular size and Lewis acid–base properties. The DESs with a pH value close to 7, a smaller molecular size, and a higher Lewis acidity yielded PICs with a higher Mn. The formation of hydrogen bonds between the DES and the monomer was conducive to the preparation of high-molecular-weight PIC. Among all DES catalysts, the synthesis of PIC catalyzed by choline chloride–ethylene glycol (n:n = 1:2) had the highest Mn, reaching 116,100 g·mol–1. Obtained PIC maintained a high transmittance (>90%) and low yellowness (b* < 0.40). Mechanistic study demonstrated the synergistic activation of ISB and DPC by hydrogen bonding sites of the hydrogen bonding acceptor and hydrogen bonding donor in the DES to prepare PIC. This strategy not only provides a low-cost and efficient method for synthesizing PIC but also offers catalyst design guidance for hydroxyl activation.
使用无金属深共晶溶剂制备高分子量生物基聚碳酸酯:羟基的高效活化
活化单体中的羟基对于制备高分子量生物来源聚碳酸酯至关重要。在此,我们开发了无金属深共晶溶剂(DESs),通过氢键相互作用激活异山梨醇(ISB)中的内羟基和外羟基,从而促进与碳酸二苯酯(DPC)通过熔融缩聚制备聚(碳酸异山梨酯)(PIC)。催化评估结果表明,与分子大小和路易斯酸碱特性相比,DES 的布氏酸碱特性对 PIC 的锰有更大的影响。pH 值接近 7、分子尺寸较小且路易斯酸度较高的 DES 生成的 PIC 锰含量较高。DES 与单体之间形成氢键有利于制备高分子量的 PIC。在所有 DES 催化剂中,氯化胆碱-乙二醇(n:n = 1:2)催化合成的 PIC 的 Mn 最高,达到 116,100 g-mol-1。获得的 PIC 保持了较高的透光率(90%)和较低的黄度(b* <0.40)。机理研究表明,DES 中的氢键受体和氢键供体的氢键位点能协同激活 ISB 和 DPC,从而制备出 PIC。该策略不仅为合成 PIC 提供了一种低成本、高效率的方法,还为羟基活化提供了催化剂设计指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
文献相关原料
公司名称
产品信息
麦克林
glycerol (Gl)
麦克林
triethanolamine (TEA)
阿拉丁
Choline chloride (ChCl)
阿拉丁
ethylene glycol (EG)
阿拉丁
1,3-propanediol (PDO)
阿拉丁
1,6-hexanediol
阿拉丁
1,10-decanediol
阿拉丁
ethanolamine
阿拉丁
diethanolamine (DEA)
阿拉丁
diphenyl carbonate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


