Practical One-Pot Synthesis of 2-Alkyl-Substituted Benzothiazoles from Bis-(2-nitrophenyl)-disulfides
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
2-Methyl benzothiazoles are widely used as key precursors for dyes, photosensitizers, and fluorescent markers. Thus, they are demanded in multigram and even kilogram quantities. We propose a scalable single-step procedure for producing 2-alkyl-substituted benzothiazoles from the corresponding bis-(2-nitrophenyl)-disulfide commercial, technical-grade quality. The reaction scope looks promising; substrates containing various substituents, including carboxylic and ester groups, were introduced. The reaction conditions were carefully optimized according to reducing agents (diverse sodium salts), solvents, ratio, and reaction time. This led to the acquisition of target products in up to 350 g quantities in a single run.
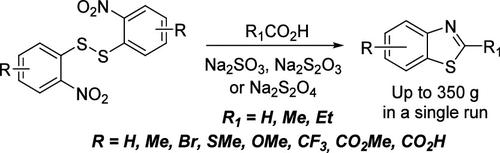
从双-(2-硝基苯基)二硫化物单锅合成 2-烷基取代的苯并噻唑的实用方法
2 甲基苯并噻唑被广泛用作染料、光敏剂和荧光标记的关键前体。因此,它们的需求量高达几克甚至几千克。我们提出了一种可扩展的单步程序,用于从相应的双(2-硝基苯基)二硫化物中生产出商业技术级质量的 2-烷基取代的苯并噻唑。反应范围看起来很有前景;引入了含有各种取代基(包括羧基和酯基)的底物。根据还原剂(各种钠盐)、溶剂、比例和反应时间,对反应条件进行了仔细优化。这样,一次运行就能获得多达 350 克的目标产品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


