Transgelin 2 guards T cell lipid metabolism and antitumour function
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
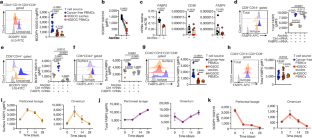
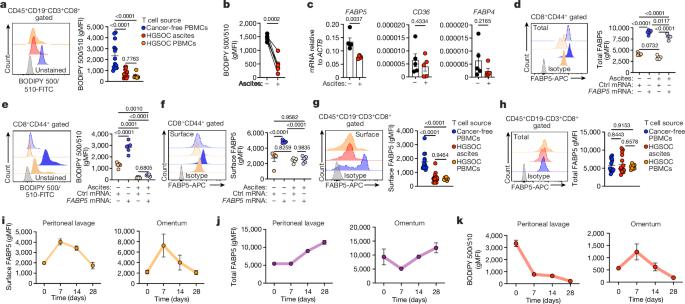
Mounting effective immunity against pathogens and tumours relies on the successful metabolic programming of T cells by extracellular fatty acids1–3. Fatty-acid-binding protein 5 (FABP5) has a key role in this process by coordinating the efficient import and trafficking of lipids that fuel mitochondrial respiration to sustain the bioenergetic requirements of protective CD8+ T cells4,5. However, the mechanisms that govern this immunometabolic axis remain unexplored. Here we report that the cytoskeletal organizer transgelin 2 (TAGLN2) is necessary for optimal fatty acid uptake, mitochondrial respiration and anticancer function in CD8+ T cells. TAGLN2 interacts with FABP5 to facilitate its cell surface localization and function in activated CD8+ T cells. Analyses of ovarian cancer specimens revealed that endoplasmic reticulum (ER) stress responses induced by the tumour microenvironment repress TAGLN2 in infiltrating CD8+ T cells, thereby enforcing their dysfunctional state. Restoring TAGLN2 expression in ER-stressed CD8+ T cells increased their lipid uptake, mitochondrial respiration and cytotoxic capacity. Accordingly, chimeric antigen receptor T cells overexpressing TAGLN2 bypassed the detrimental effects of tumour-induced ER stress and demonstrated therapeutic efficacy in mice with metastatic ovarian cancer. Our study establishes the role of cytoskeletal TAGLN2 in T cell lipid metabolism and highlights the potential to enhance cellular immunotherapy in solid malignancies by preserving the TAGLN2–FABP5 axis. Together with the fatty-acid-binding protein FABP5, the cytoskeletal organizer TAGLN2 is an essential factor for fatty acid uptake, mitochondrial respiration and anticancer function in CD8+ T cells.
转铁蛋白 2 保护 T 细胞脂质代谢和抗肿瘤功能
针对病原体和肿瘤的有效免疫依赖于细胞外脂肪酸对 T 细胞的成功代谢编程1,2,3。脂肪酸结合蛋白 5 (FABP5) 在这一过程中起着关键作用,它协调脂质的有效输入和运输,为线粒体呼吸提供燃料,以维持保护性 CD8+ T 细胞的生物能需求4,5。然而,支配这一免疫代谢轴的机制仍有待探索。在这里,我们报告了细胞骨架组织者转铁蛋白 2(TAGLN2)对于 CD8+ T 细胞的最佳脂肪酸摄取、线粒体呼吸和抗癌功能是必要的。TAGLN2 与 FABP5 相互作用,促进其在活化的 CD8+ T 细胞中的细胞表面定位和功能。对卵巢癌标本的分析表明,肿瘤微环境诱导的内质网(ER)应激反应抑制了浸润CD8+ T细胞中的TAGLN2,从而强化了它们的功能障碍状态。在ER应激的CD8+ T细胞中恢复TAGLN2的表达可增加它们的脂质摄取、线粒体呼吸和细胞毒性能力。因此,过表达 TAGLN2 的嵌合抗原受体 T 细胞绕过了肿瘤诱导的 ER 应激的有害影响,并对转移性卵巢癌小鼠显示出疗效。我们的研究确定了细胞骨架 TAGLN2 在 T 细胞脂质代谢中的作用,并强调了通过保留 TAGLN2-FABP5 轴来增强实体恶性肿瘤细胞免疫疗法的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


