Chromatin remodelling drives immune cell–fibroblast communication in heart failure
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
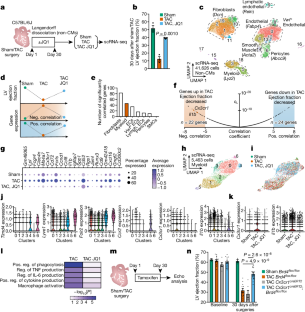
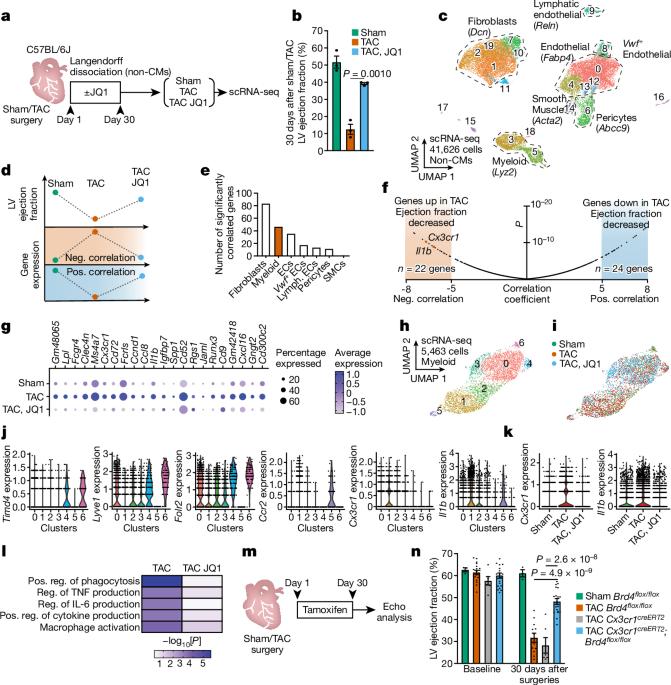
Chronic inflammation and tissue fibrosis are common responses that worsen organ function, yet the molecular mechanisms governing their cross-talk are poorly understood. In diseased organs, stress-induced gene expression changes fuel maladaptive cell state transitions1 and pathological interaction between cellular compartments. Although chronic fibroblast activation worsens dysfunction in the lungs, liver, kidneys and heart, and exacerbates many cancers2, the stress-sensing mechanisms initiating transcriptional activation of fibroblasts are poorly understood. Here we show that conditional deletion of the transcriptional co-activator Brd4 in infiltrating Cx3cr1+ macrophages ameliorates heart failure in mice and significantly reduces fibroblast activation. Analysis of single-cell chromatin accessibility and BRD4 occupancy in vivo in Cx3cr1+ cells identified a large enhancer proximal to interleukin-1β (IL-1β, encoded by Il1b), and a series of CRISPR-based deletions revealed the precise stress-dependent regulatory element that controls Il1b expression. Secreted IL-1β activated a fibroblast RELA-dependent (also known as p65) enhancer near the transcription factor MEOX1, resulting in a profibrotic response in human cardiac fibroblasts. In vivo, antibody-mediated IL-1β neutralization improved cardiac function and tissue fibrosis in heart failure. Systemic IL-1β inhibition or targeted Il1b deletion in Cx3cr1+ cells prevented stress-induced Meox1 expression and fibroblast activation. The elucidation of BRD4-dependent cross-talk between a specific immune cell subset and fibroblasts through IL-1β reveals how inflammation drives profibrotic cell states and supports strategies that modulate this process in heart disease and other chronic inflammatory disorders featuring tissue remodelling. Conditional deletion of the transcriptional co-activator Brd4 in infiltrating Cx3cr1+ mouse macrophages ameliorates heart failure and substantially reduces fibroblast activation.
染色质重塑推动心力衰竭中免疫细胞与成纤维细胞的交流
慢性炎症和组织纤维化是导致器官功能恶化的常见反应,但人们对其相互影响的分子机制却知之甚少。在患病器官中,应激诱导的基因表达变化会助长不适应的细胞状态转换1 和细胞间的病理相互作用。尽管成纤维细胞的慢性活化会加重肺、肝、肾和心脏的功能障碍,并使许多癌症恶化2,但人们对启动成纤维细胞转录活化的应激感应机制知之甚少。在这里,我们发现有条件地删除浸润 Cx3cr1+ 巨噬细胞中的转录共激活因子 Brd4 可改善小鼠的心力衰竭,并显著降低成纤维细胞的激活。对 Cx3cr1+ 细胞中单细胞染色质可及性和体内 BRD4 占有率的分析确定了白细胞介素-1β(IL-1β,由 Il1b 编码)近端的大型增强子,一系列基于 CRISPR 的缺失揭示了控制 Il1b 表达的精确应激依赖性调控元件。分泌的IL-1β激活了转录因子MEOX1附近的成纤维细胞RELA依赖性(也称为p65)增强子,导致人类心脏成纤维细胞的损伤性反应。在体内,抗体介导的 IL-1β 中和可改善心衰患者的心脏功能和组织纤维化。全身抑制IL-1β或在Cx3cr1+细胞中靶向删除Il1b可防止应激诱导的Meox1表达和成纤维细胞活化。通过IL-1β阐明特定免疫细胞亚群与成纤维细胞之间依赖于BRD4的交叉对话,揭示了炎症是如何驱动坏死细胞状态的,并支持在心脏病和其他以组织重塑为特征的慢性炎症性疾病中调节这一过程的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


