An assessment of electroneutrality implementations for accurate electrochemical ion transport models
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
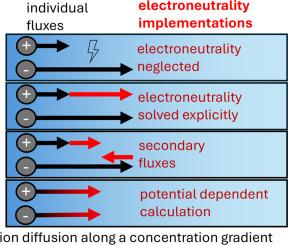
During the diffusion and migration of ions in electrolytes, the electrodynamic ion-ion interactions prevent charge separation despite different ionic mobilities, ultimately enforcing electroneutrality in the bulk electrolyte. To model ion transport accurately, a method to enforce electroneutrality must be implemented. In this study, four strategies to implement electroneutrality are discussed and evaluated. The ion distributions that result from a transport model with the different electroneutrality implementations are calculated, considering various electrolytes and sets of electrochemical parameters. The meaningfulness and applicability of each implementation are assessed through spatial charge accumulations, transference numbers, and experimental data from the literature. Combining the electrochemical ion transport models with the electroneutrality constraint for all ions is shown to result in an overdetermined system of equations if the driving forces are calculated under neglection of diffusion potentials. The often-reported model simplification of using the electroneutrality constraint to resolve the transport of one specific species explicitly results in non-physically correct mass transport. A practical approach to precisely describe the measured physicochemical ion movements is obtained by equilibrating spatial charges with the ion conduction for every time step in the ion transport model, which is reasonably applicable to multi-ion systems in three-dimensional frameworks. This comprehensive assessment aims to guide readers in selecting an appropriate electroneutrality implementation framework for ion transport models.
评估精确电化学离子传输模型的电中性实施情况
在电解质中离子的扩散和迁移过程中,尽管离子的迁移率不同,但电动离子间的相互作用阻止了电荷分离,最终在大体积电解质中实现了电中性。为了准确地模拟离子传输,必须采用一种方法来实现电中性。本研究讨论并评估了实现电中性的四种策略。考虑到各种电解质和电化学参数集,计算了采用不同电中性实施方法的传输模型所产生的离子分布。通过空间电荷累积、转移数和文献中的实验数据,对每种实现方式的意义和适用性进行了评估。将电化学离子输运模型与所有离子的电中性约束相结合,如果在负选择扩散电位下计算驱动力,则会导致过确定方程组。经常报道的使用电中性约束来解决某一特定物种迁移的模型简化会导致非物理正确的质量迁移。在离子输运模型中,通过平衡每一个时间步的空间电荷与离子传导,可以获得精确描述测量到的物理化学离子运动的实用方法,该方法合理地适用于三维框架中的多离子系统。本综合评估旨在指导读者为离子输运模型选择合适的电中性实施框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


