A Select thiosemicarbazone copper(II) complex induces apoptosis in Gastric Cancer and targets Cancer Stem Cells reducing pluripotency markers.
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
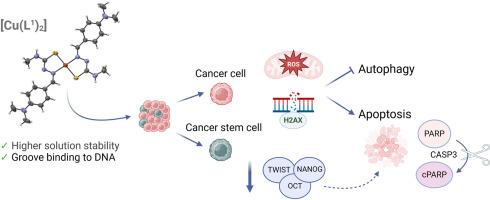
Copper(II)-based complexes are promising candidates as anti-cancer agents due to their ability to target cancer cells. Here we describe the synthesis and characterization of two copper(II) thiosemicarbazone complexes with the ligands 4-(dimethylamino)benzaldehyde N4-methylthiosemicarbazone (HL1) and 4-(dimethylamino)benzaldehyde N4-(4-(dimethylamino)phenylthiosemicarbazone (HL2) and general formula [Cu(L)2]. The complexes show stability in aqueous solution with 1% of DMSO that allows to stablish its solution profile in biological buffers. Compound [Cu(L1)₂] lipophilicity was lower than [Cu(L2)₂] however its solubility in biological buffer was not only better but also its DLS and z-potencial data. In vitro studies demonstrate a higher cytotoxic effect of [Cu(L1)₂] on gastric cancer cells. The proposed mechanism of action consists in the generation of free radicals that induce DNA lesions, oxidative stress and ultimately autophagy deregulation and apoptosis. Additionally, [Cu(L1)₂] is equally active on gastric cancer stem cells and tumor cells resistant to cisplatin. More importantly, stem cells treated with [Cu(L1)₂] show a downregulation of pluripotency markers such as TWIST, NANOG and OCT4. Overall, our results with [Cu(L1)₂] prompt a significant advancement in the development of rational-designed pharmaceuticals for combating cancer.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


