Role of Graphite Electrodes for Removal of Persistent Contaminants in Electrochemical Systems with Chloride Salts as an Electrolyte: A Case Study of Disappearance of an Azo Dye
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
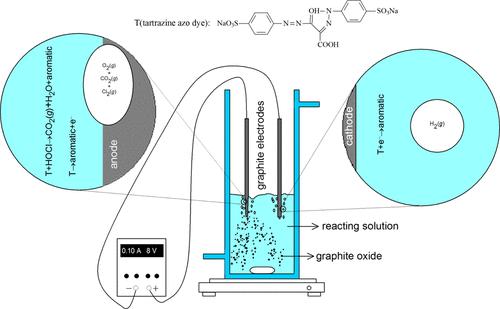
In this study, a Hoffman cell with a 4.28 M NaCl solution at 298 K and initial pH ∼ 6 was operated at 0.1 A and ∼16 V to identify the main redox reactions on the surface of the graphite anode and cathode. The measured volumes of evolved gas and the concentration of free chlorine in the anode cell revealed that H2O, graphite, and Cl– were oxidized to form O2(g), CO2(g), and Cl2(g) at current efficiencies accurately determined. The recorded volume of gas at the cathode indicated that the major reaction in this cell was the electrochemical (EC) reduction of H2O to form H2(g) at a current efficiency close to unity. Experiments of consumption of tartrazine azo dye with graphite electrodes were also carried out in reactors with divided and undivided EC cells at potentials ≥8 V, at different NaCl concentrations (2.74, 5.13 × 10–3 and 2.57 × 10–3 M) and currents (0.1 and 0.2 A). Tartrazine was almost completely removed from the solution by both anodic oxidation (direct and indirect) and cathodic reduction and at times markedly decreased as the concentration of NaCl and current increased. The kinetics of tartrazine consumption, pH change, and anode consumption under all the considered conditions was properly described by a model involving heterogeneous and homogeneous reactions. The main innovative aspect of the model was the suggested individual rate expressions for the in situ electrogeneration of O2(g), CO2(g), and Cl2(g) on the surface of graphite anodes as a function of the concentration of Cl–. Scanning electron microscopy/energy-dispersive X-ray, total organic carbon, and spectrophotometric analyses confirmed graphite oxide as one of the products of C(s) oxidation at the anode, and aromatic species, with CO2(g) and H2O in low yields, as products of the redox reactions of tartrazine.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


