Inhibition of α-Synuclein Misfolding into β-Sheet Domains on Medium-Sized Gold Nanoclusters: Evidence from Enhanced Sampling MD Simulations
IF 5.2
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
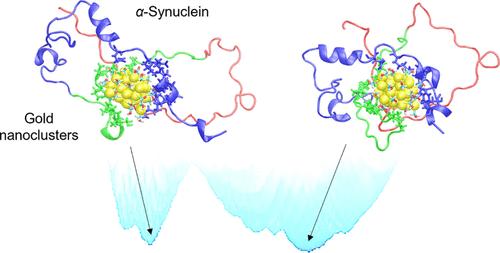
Targeting Parkinson’s disease (PD) related protein, α-synuclein (αS), via gold nanoclusters (AuNCs) has received considerable attention in PD treatments, but its molecular basis on the initial interactions between αS and AuNCs remains elusive due to the absence of a unique secondary structure of αS chains. Here, at the single-cluster level, we incorporate well-tempered metadynamics simulations to explore the structural and thermodynamic characteristics of the full length αS adsorbed on different-sized AuNCs (Aun, n = 25, 36, 44, 68, 102) with modeled thiolated ligands (Aun@Lig). The conformational landscapes of αS indicate that uncharged Aun@SCH2OH chaperones the native intrinsically disordered conformations of αS, while negatively and positively charged AuNCs greatly increase the likelihood of forming intramolecular β-sheet domains, which are necessary for αS fibrillation and are a hallmark of PD. The binding details further demonstrate the significant inhibitory effect of the medium-sized Au36@SCH2OH on αS misfolding into β-sheet domains. This provides a valuable guideline for customizing AuNCs to precisely manipulate protein folding and misfolding behaviors, with potential implications for disease treatments.
中等尺寸金纳米团簇抑制α-突触核蛋白错折叠成β片状结构域:来自增强采样 MD 模拟的证据
通过金纳米团簇(AuNCs)靶向帕金森病(PD)相关蛋白α-突触核蛋白(αS)已在帕金森病治疗中受到广泛关注,但由于αS链缺乏独特的二级结构,αS与AuNCs之间初始相互作用的分子基础仍然难以捉摸。在此,我们在单团簇水平上进行了恒定元动力学模拟,以探索吸附在不同大小 AuNCs(Aun,n = 25、36、44、68、102)上的全长 αS 与模型硫醇配体(Aun@Lig)的结构和热力学特征。αS的构象图谱表明,不带电的Aun@SCH2OH会使αS的原生固有无序构象发生变化,而带负电和正电的AuNCs则会大大增加形成分子内β片状结构域的可能性,而β片状结构域是αS纤维化的必要条件,也是PD的标志。结合细节进一步证明了中等大小的 Au36@SCH2OH 对 αS 错折叠成 β 片状结构域有显著的抑制作用。这为定制 AuNCs 以精确操纵蛋白质折叠和错误折叠行为提供了宝贵的指导,对疾病治疗具有潜在的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


