The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Spleen tyrosine kinase (SYK) is central to adaptive and innate immune signaling. It features a regulatory region containing tandem SH2 (tSH2) domains separated by a helical “hinge” segment keeping SYK inactive by associating with the kinase domain. SYK activation is triggered when the tSH2 domains bind to a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) found on receptor tails. Past mutational studies have indicated that ITAM binding disrupts the hinge-kinase interaction, leading to SYK phosphorylation and activation. However, the mechanism of this process is unclear, as the ITAM interaction occurs far from the hinge region. We have determined crystal structures of three phospho-ITAMs in complex with the tSH2 domains, revealing a highly conserved binding mechanism. These structures, together with mutational studies and biophysical analyses, reveal that phospho-ITAM binding restricts SH2 domain movement and causes allosteric changes in the hinge region. These changes are not compatible with the association of the kinase domain, leading to kinase activation.
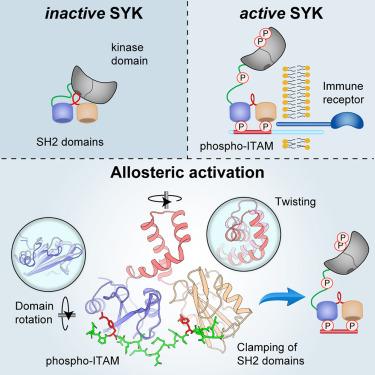
从多个磷酸-ITAM 结合结构得出的 SYK 激酶异位激活机制
脾酪氨酸激酶(SYK)是适应性免疫和先天性免疫信号传导的核心。它的调节区包含串联 SH2(tSH2)结构域,由螺旋 "铰链 "区段分隔,通过与激酶结构域结合保持 SYK 的非活性。当 tSH2 结构域与受体尾部的磷酸化免疫受体酪氨酸激活基团(ITAM)结合时,就会触发 SYK 激活。过去的突变研究表明,ITAM 结合会破坏铰链与激酶的相互作用,导致 SYK 磷酸化和激活。然而,这一过程的机制尚不清楚,因为 ITAM 的相互作用发生在远离铰链区的地方。我们测定了三种磷酸化 ITAM 与 tSH2 结构域复合物的晶体结构,揭示了一种高度保守的结合机制。这些结构以及突变研究和生物物理分析表明,磷酸化 ITAM 结合会限制 SH2 结构域的移动,并导致铰链区的异构变化。这些变化与激酶结构域的结合不相容,从而导致激酶活化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


