Adipocyte-derived glutathione promotes obesity-related breast cancer by regulating the SCARB2-ARF1-mTORC1 complex
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Obesity is a major risk factor for poor breast cancer outcomes, but the impact of obesity-induced tumor microenvironment (TME) metabolites on breast cancer growth and metastasis remains unclear. Here, we performed TME metabolomic analysis in high-fat diet (HFD) mouse models and found that glutathione (GSH) levels were elevated in the TME of obesity-accelerated breast cancer. The deletion of glutamate-cysteine ligase catalytic subunit (GCLC), the rate-limiting enzyme in GSH biosynthesis, in adipocytes but not tumor cells reduced obesity-related tumor progression. Mechanistically, we identified that GSH entered tumor cells and directly bound to lysosomal integral membrane protein-2 (scavenger receptor class B, member 2 [SCARB2]), interfering with the interaction between its N and C termini. This, in turn, recruited mTORC1 to lysosomes through ARF1, leading to the activation of mTOR signaling. Overall, we demonstrated that GSH links obesity and breast cancer progression by acting as an activator of mTOR signaling. Targeting the GSH/SCARB2/mTOR axis could benefit breast cancer patients with obesity.
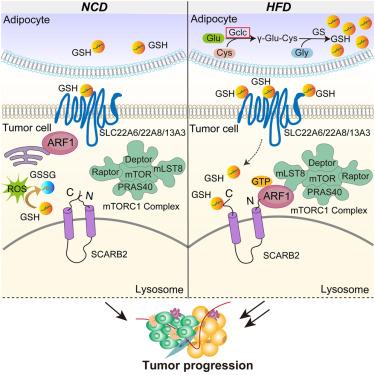
源于脂肪细胞的谷胱甘肽通过调节 SCARB2-ARF1-mTORC1 复合物促进肥胖相关乳腺癌的发生
肥胖是导致乳腺癌预后不良的一个主要风险因素,但肥胖引起的肿瘤微环境(TME)代谢物对乳腺癌生长和转移的影响仍不清楚。在这里,我们在高脂饮食(HFD)小鼠模型中进行了TME代谢组学分析,发现在肥胖加速的乳腺癌TME中谷胱甘肽(GSH)水平升高。脂肪细胞中谷胱甘肽-半胱氨酸连接酶催化亚基(GCLC)是谷胱甘肽生物合成的限速酶,而肿瘤细胞中GCLC的缺失可减轻肥胖相关的肿瘤进展。从机理上讲,我们发现 GSH 进入肿瘤细胞后会直接与溶酶体完整膜蛋白-2(清道夫受体 B 类成员 2 [SCARB2])结合,干扰其 N 端和 C 端之间的相互作用。这反过来又通过 ARF1 将 mTORC1 募集到溶酶体,导致 mTOR 信号的激活。总之,我们证明了 GSH 通过作为 mTOR 信号转导的激活剂将肥胖与乳腺癌的进展联系在一起。靶向GSH/SCARB2/mTOR轴可使肥胖症乳腺癌患者获益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


