Electrochemical CO2 fixation with amines to synthesize α-amino acids
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
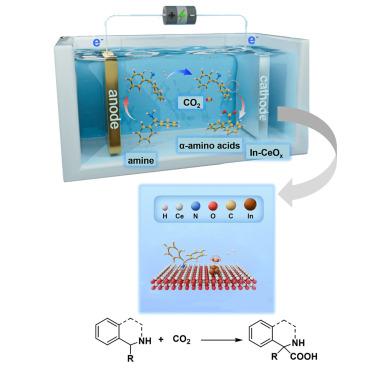
α-Amino acids (α-aa) play a significant role in pharmaceutical and chemical industries. Here, we reported an electrochemical tandem system that couples the anodic dehydrogenation of amines and the cathodic carboxylation of imines. Based on a designed Lewis acid (LA)-enriched CeO2 with indium (denoted as In-CeOx) as cathodic electrocatalysts, we achieved 82% and 92% yields of stable imines and α-aa in a membrane-separated cell system, respectively. In a membrane-free system, quaternary or cyclic α-aa could be directly obtained from amines and CO2 with up to 83% yield. Mechanistic investigations have elucidated that the incorporation of indium (In) yields elevated levels of LA sites. These enhanced LA sites play a pivotal role in facilitating the capture and activation of imines. This function of In-CeOx, coupled with CO2 activation mediated by In species, is proven to be crucial for achieving high reactivity and selectivity in the cathodic carboxylation reaction.
用胺进行电化学二氧化碳固定以合成α-氨基酸
α-氨基酸(α-aa)在制药和化学工业中发挥着重要作用。在此,我们报告了一种将胺的阳极脱氢和亚胺的阴极羧化耦合在一起的电化学串联系统。基于设计的富含路易斯酸(LA)和铟(In-CeOx)的 CeO2 作为阴极电催化剂,我们在膜分离电池系统中分别获得了 82% 和 92% 的稳定亚胺和 α-aa 产率。在无膜系统中,可直接从胺和二氧化碳中获得四元或环状 α-aa,产率高达 83%。机理研究阐明,铟(In)的加入会产生更多的 LA 位点。这些增强的 LA 位点在促进亚胺的捕获和活化方面发挥了关键作用。事实证明,In-CeOx 的这一功能,加上 In 物种介导的二氧化碳活化,对于在阴极羧化反应中实现高反应性和选择性至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


