Harnessing biological insights: Integrating proton regulation, pH buffer and zincophility for highly stable Zn anode
IF 16.8
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Zinc anodes are severely threatened by hydrogen evolution, dendrite growth and by-products. Inspired by the mechanisms of L-carnosine (L-car) in maintaining intracellular pH stability and fostering mucosal repair in biological systems, this study innovatively proposed a comprehensive strategy integrating proton regulation, pH buffer and zincophility by introducing L-car as an electrolyte additive to achieve remarkable stability of zinc anode. Experimental validations and theoretical calculations demonstrate that the abundant N and O sites in L-car can form robust hydrogen bonds with protons, effectively impeding interfacial proton transport and substantially mitigating HER. Moreover, dual pH buffering sites of L-car facilitate dynamic modulation of proton concentration, stabilizing the pH of electrolyte, and suppressing Zn corrosion/passivation/by-product. Concurrently, the robust chelation between L-car and Zn2+ orchestrates uniform zinc ion deposition. This synergistic trifecta of L-car endows Zn ion batteries with an extraordinary cycling stability, extending to an ultralong duration of 5500 h. Furthermore, the Zn//MnO2 full battery, demonstrates remarkable stability, retaining a specific capacity of 106.1 mAh/g with 79.2 % retention after 1000 cycles at 3 A/g. This study pioneers the interdisciplinary application of L-car in electrolyte modification, presenting a novel paradigm for stabilizing zinc anodes.
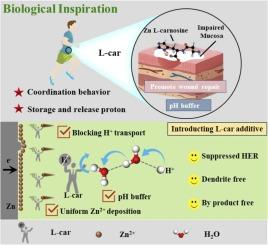
利用生物学洞察力:整合质子调节、pH 缓冲和亲锌性,实现高度稳定的锌阳极
锌阳极受到氢演化、枝晶生长和副产品的严重威胁。受左旋肉碱(L-car)在生物系统中维持细胞内 pH 值稳定和促进粘膜修复机制的启发,本研究创新性地提出了一种集质子调节、pH 缓冲和锌亲和性于一体的综合策略,即引入左旋car 作为电解质添加剂,以实现锌阳极的显著稳定性。实验验证和理论计算证明,L-car 中丰富的 N 和 O 位点可与质子形成牢固的氢键,有效阻碍界面质子传输,大大减轻 HER。此外,L-car 的双 pH 缓冲位点有助于动态调节质子浓度,稳定电解质的 pH 值,抑制锌的腐蚀/钝化/副产物。同时,L-car 与 Zn2+ 之间的强螯合作用还能协调锌离子的均匀沉积。L-car 的这三重协同作用使锌离子电池 (ZIB) 具有超强的循环稳定性,可持续 5500 小时的超长时间。此外,Zn//MnO2 全电池表现出卓越的稳定性,在 3 A/g 条件下循环 1000 次后,比容量仍保持在 106.1 mAh/g,保留率为 79.2%。这项研究开创了 L-car 在电解质改性方面的跨学科应用,为稳定锌阳极提供了一种新的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


