Discovery and Synthesis of Heterobifunctional Degraders of Rearranged during Transfection (RET) Kinase
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
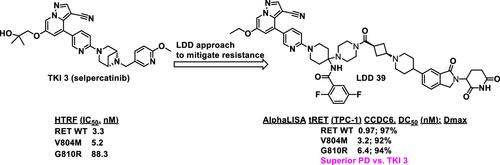
We describe the design, synthesis, and structure–activity relationship (SAR) of heterobifunctional RET ligand-directed degraders (LDDs) derived from three different second-generation RET inhibitors. These LDDs are composed of a target binding motif (TBM) that binds to the RET protein, a linker, and a cereblon binding motif (CBM) as the E3 ligase recognition unit. This led to the identification of a series of pyrazolopyridine-based heterobifunctional LDDs, as exemplified by compound 39. LDD 39 demonstrated high in vitro inhibitory and degradation potency against both RET wild-type and the two representative mutants, V804M and G810R. Importantly, in PK/PD studies, 39 exhibited a differentiated and favorable in vivo profile compared to the corresponding tyrosine kinase inhibitor (TKI), compound 3. Robust and sustained degradation of total-RET (tRET) protein and inhibition of phospho-RET (pRET) signaling were observed in TPC-1 xenograft tumors driven by RET and the RET/G810R mutant following a single dose of LDD 39 at 15 and 75 mg/kg, respectively.
发现和合成转染过程中重新排列(RET)激酶的异多功能降解剂
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


