Hydroxyl group dynamics in defernite: Raman spectroscopy studies
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-10-16
DOI:10.1016/j.saa.2024.125289
引用次数: 0
Abstract
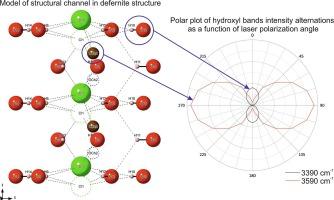
A detailed examination of the altered silicate-carbonate xenolith embedded within the ignimbrite of the Upper Chegem Caldera revealed a new occurrence of a rare carbonate mineral known as defernite, with chemical formula Ca6[(CO3)2-x(Si2O7)x/2](OH)7[Cl1-x(H2O)x], where x ≈ 0.4. Defernite crystallizes as colorless to white fibrous aggregates, reaching 100–150 μm diameters. Subsequently, Raman investigations of defernite from the Upper Chegem Caldera were conducted to perform a comprehensive structural analysis and compare it with minerals found in other locations. During this examination, band assignments focused on the carbonate ion vibration (CO32−) with a band at 1085 cm−1 and the hydroxyl group, characterized by a series of strong bands around 3590–3600 cm−1, particularly evident in oriented crystals along the (0 1 0) plane. Experimentation involving the alteration of incident laser light polarization highlighted a reduction in the intensity of carbonate and hydroxyl-related bands and the activation of a band around 3390 cm−1. This phenomenon is explained by the formation of hydrogen bonding between hydroxyl groups and chlorine or molecular water, potentially occupying chlorine positions. Lastly, a temperature-dependent experiment demonstrated the instability of the 3390 cm−1 band, which dissipated with increasing temperature. This insight explains the band’s origin around 3590 cm−1, ascribed to non-degenerate hydroxyl groups as a key marker within the defernite structure.
角闪石中羟基的动力学:拉曼光谱研究。
对嵌在上切格姆破火山口火成岩中的蚀变硅酸盐-碳酸盐异质岩进行的详细研究发现了一种新的稀有碳酸盐矿物--铁闪石,其化学式为Ca6[(CO3)2-x(Si2O7)x/2](OH)7[Cl1-x(H2O)x],其中x≈0.4。白铁矿结晶为无色至白色纤维状聚集体,直径可达 100-150 μm。随后,对上切格姆破火山口的铁闪石进行了拉曼研究,以进行全面的结构分析,并将其与在其他地方发现的矿物进行比较。在这次研究中,波段分配的重点是碳酸根离子振动(CO32-)(波段在 1085 cm-1 处)和羟基(在 3590-3600 cm-1 附近有一系列强波段,在沿 (010) 平面的定向晶体中尤为明显)。改变入射激光偏振的实验结果表明,碳酸盐和羟基相关波段的强度降低,3390 cm-1 附近的波段被激活。这一现象的原因是羟基与氯或分子水之间形成了氢键,可能占据了氯的位置。最后,与温度相关的实验证明了 3390 cm-1 波段的不稳定性,它随着温度的升高而消失。这一发现解释了 3590 厘米-1 附近频带的来源,即非退化羟基是脱苯乙烯结构中的一个关键标记。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


