Metal-organic-framework and walnut shell biochar composites for lead and hexavalent chromium removal from aqueous environments
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Extensive research in recent years has explored the realm of porous carbon composites for various applications, including electrochemistry, structural materials, environmental remediation, and more. In particular, the fabrication of porous carbon composites using a metal-organic framework (MOF) and biochar (BC) for aqueous remediation is a fairly new avenue of research. In this study, a MOF-BC composite was synthesized with unmodified and chemically modified BCs using solvothermal synthesis. The composites were used as adsorbents to remediate heavy metals, such as lead (II) and chromium (VI), from aqueous environments. It was verified that the MOF was homogeneously deposited onto the BC's surface using various material characterization techniques. Lead and chromium adsorption studies revealed a high adsorption capacity with greater than 99% removal for lead and ∼65% for chromium, respectively. Impressively, for lead, the highest observed experimental adsorption capacity of the MOF-chemically modified BC composite was 535 mg/g, compared to 240 mg/g for pristine BC. Meanwhile, the adsorption capacity of the same MOF-BC composite for chromium ions was low at 18 mg/g, compared to 80 mg/g for the chemically modified BC. The MOF-BC had a rapid adsorption rate, achieving equilibrium at only 150 min of reaction time for lead ions. MOF-BCs have higher adsorption for cationic lead through physisorption and ion-exchange mechanisms, whereas, for anionic chromium, removal is dominated only by physisorption mechanisms. The outcomes and methodological developments attained in this study offer a novel and compelling approach for synthesizing MOF-BC composites for aqueous remediation applications.
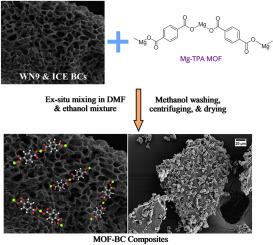
金属有机框架和核桃壳生物炭复合材料用于去除水环境中的铅和六价铬。
近年来的大量研究探索了多孔碳复合材料的各种应用领域,包括电化学、结构材料、环境修复等。其中,利用金属有机框架(MOF)和生物炭(BC)制造多孔碳复合材料用于水体修复是一个相当新的研究方向。本研究采用溶热合成法合成了一种 MOF-BC 复合材料,其中包括未改性和化学改性的生物炭。该复合材料被用作吸附剂,以去除水环境中的铅(II)和铬(VI)等重金属。利用各种材料表征技术验证了 MOF 在 BC 表面的均匀沉积。铅和铬的吸附研究表明,MOF 具有很高的吸附能力,对铅和铬的去除率分别超过 99% 和 65%。令人印象深刻的是,对于铅,MOF-化学修饰 BC 复合材料的最高实验吸附容量为 535 mg/g,而原始 BC 为 240 mg/g。同时,同一种 MOF-BC 复合材料对铬离子的吸附容量较低,仅为 18 毫克/克,而化学修饰 BC 的吸附容量为 80 毫克/克。MOF-BC 的吸附速度很快,对铅离子的吸附仅在 150 分钟的反应时间内就达到了平衡。MOF-BC 通过物理吸附和离子交换机制对阳离子铅具有较高的吸附能力,而对阴离子铬的去除仅以物理吸附机制为主。本研究的成果和方法论的发展为合成 MOF-BC 复合材料用于水体修复应用提供了一种新颖而有吸引力的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


