Bioengineering strategy to promote CNS nerve growth and regeneration via chronic glutamate signaling
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
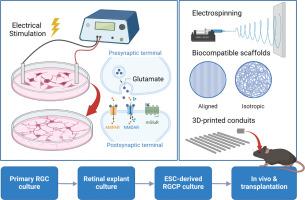
Being part of the mature mammalian central nervous system, impairments of the retina and optic nerves caused by trauma or diseases often cannot be restored. Progressive degeneration of retinal ganglion cells (RGCs) in glaucoma and other optic neuropathies gradually leads to permanent vision loss, which currently has no cure. The purpose of this study is to develop a biocompatible scaffold to support RGC survival and guide axon growth, facilitating optic nerve repair and regeneration. We here report that electrical stimulation (ES) significantly promoted neurite outgrowth and elongation from primary RGCs, mediated through glutamate receptor signaling. To mimic prolonged glutamate stimulation and facilitate sustained nerve growth, we fabricated biocompatible poly-γ-benzyl-L-glutamate (PBG) scaffolds for controlled glutamate release. These PBG scaffolds supported RGC survival and robust long-distance nerve growth in both retinal explants and isolated RGC cultures. In contrast, control polycaprolactone (PCL) scaffolds with similar physical structures showed little benefits on RGC survival or nerve growth. Moreover, PBG scaffolds promoted the differentiation and neurite outgrowth from embryonic stem cell-derived RGC progenitors. The aligned PBG scaffold drove directed nerve elongation along the fiber alignment. Transplantation of PBG-coated biocompatible conduits induced robust optic nerve regeneration in adult mice following nerve transection. Together, the findings present the exciting possibility of driving optic nerve regeneration and RGC progenitor cell differentiation by imitating ES or glutamate signaling. PBG presents a permissive biomaterial in supporting robust and directed axon growth with promising clinical applications in the future.
Statement of Significance
We here reported compelling findings that demonstrate the potent regenerative effects of a bioengineered scaffold incorporating poly-γ-benzyl-L-glutamate (PBG) on the optic nerve. Retinal ganglion cell (RGC) axons, which form the optic nerve, are incapable of regenerating in adulthood, posing a significant hurdle in restoring vision for patients with optic nerve diseases or injuries. Built upon the finding that electrical stimulation promotes RGC axonal growth through glutamate signaling, we developed PBG scaffolds to provide sustained glutamate stimulation and showed their exceptional effects on driving directed axonal elongation in cultured RGCs and neural progenitors, as well as supporting robust optic nerve regeneration after transection in vivo. The findings hold great promise for reversing vision loss in patients with optic nerve conditions.
通过慢性谷氨酸信号促进中枢神经系统神经生长和再生的生物工程策略
作为成熟哺乳动物中枢神经系统的一部分,视网膜和视神经因创伤或疾病造成的损伤往往无法恢复。青光眼和其他视神经病变引起的视网膜神经节细胞(RGC)逐渐退化会导致永久性视力丧失,目前尚无治疗方法。这项研究的目的是开发一种生物相容性支架,以支持 RGC 的存活并引导轴突生长,从而促进视神经的修复和再生。我们在此报告,电刺激(ES)通过谷氨酸受体信号传导,显著促进了原发性RGC的神经元外生和伸长。为了模拟长时间的谷氨酸刺激并促进神经的持续生长,我们制作了生物相容性聚-γ-苄基-L-谷氨酸(PBG)支架来控制谷氨酸的释放。在视网膜外植体和分离的 RGC 培养物中,这些 PBG 支架都能支持 RGC 的存活和长距离神经生长。相比之下,物理结构相似的聚己内酯(PCL)对照支架对 RGC 的存活或神经生长几乎没有益处。此外,PBG支架还能促进胚胎干细胞衍生的RGC祖细胞的分化和神经元的生长。排列整齐的PBG支架推动神经沿着纤维排列方向定向伸长。在成年小鼠神经横断后,移植PBG涂层的生物相容性导管可诱导强大的视神经再生。这些发现为通过模仿 ES 或谷氨酸信号驱动视神经再生和 RGC 祖细胞分化提供了令人兴奋的可能性。PBG是一种支持轴突稳健定向生长的生物材料,未来有望应用于临床。意义声明:我们在此报告了令人信服的研究结果,证明了结合聚-γ-苄基-L-谷氨酸(PBG)的生物工程支架对视神经具有强大的再生作用。构成视神经的视网膜神经节细胞(RGC)轴突在成年后无法再生,这对视神经疾病或损伤患者恢复视力构成了重大障碍。基于电刺激通过谷氨酸信号促进 RGC 轴突生长的发现,我们开发了 PBG 支架来提供持续的谷氨酸刺激,并显示了其在培养的 RGC 和神经祖细胞中驱动定向轴突生长以及在体内横断后支持强大的视神经再生方面的卓越效果。这些发现为逆转视神经疾病患者的视力丧失带来了巨大希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


