A multifunctional self-reinforced injectable hydrogel for enhancing repair of infected bone defects by simultaneously targeting macrophages, bacteria, and bone marrow stromal cells
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Injectable hydrogels (IHs) have demonstrated huge potential in promoting repair of infected bone defects (IBDs), but how to endow them with desired anti-bacterial, immunoregulatory, and osteo-inductive properties as well as avoid mechanical failure during their manipulation are challenging. In this regard, we developed a multifunctional AOHA-RA/Lap nanocomposite IH for IBDs repair, which was constructed mainly through two kinds of reversible cross-links: (i) the laponite (Lap) crystals mediated electrostatic interactions; (ii) the phenylboronic acid easter bonds between the 4-aminobenzeneboronic acid grafted oxidized hyaluronic acid (AOHA) and rosmarinic acid (RA). Due to the specific structural composition, the AOHA-RA/Lap IH demonstrated superior injectability, self-recoverability, spatial adaptation, and self-reinforced mechanical properties after being injected to the bone defect site. In addition, the RA molecules could be locally released from the hydrogel following a Weibull model for over 10 days. Systematic in vitro/vivo assays proved the strong anti-bacterial activity of the hydrogel against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). Moreover, its capability of inducing M2 polarization of macrophages (Mφ) and osteogenic differentiation of bone marrow stromal cells (BMSCs) was verified either, and the mechanism of the former was identified to be related to the JAK1-STAT1 and PI3K-AKT signaling pathways and that of the latter was identified to be related to the calcium signaling pathway, extracellular matrix (ECM) receptor interaction and TGF-β signaling pathway. After being implanted to a S. aureus infected rat skull defect model, the AOHA-RA/Lap IH significantly accelerated repair of IBDs without causing significant systemic toxicity.
Statement of significance
Rosmarinic acid and laponite were utilized to develop an injectable hydrogel, promising for accelerating repair of infected bone defects in clinic. The gelation of the hydrogel was completely driven by two kinds of reversible cross-links, which endow the hydrogel superior spatial adaption, self-recoverability, and structural stability. The as-prepared hydrogel demonstrated superior anti-bacterial/anti-biofilm activity and could induce M2 polarization of macrophages and osteogenic differentiation of BMSCs. The mechanism behind macrophages polarization was identified to be related to the JAK1-STAT1 and PI3K-AKT signaling pathways. The mechanism behind osteogenic differentiation of BMSCs was identified to be related to the ECM receptor interaction and calcium signaling/TGF-β signaling pathways.
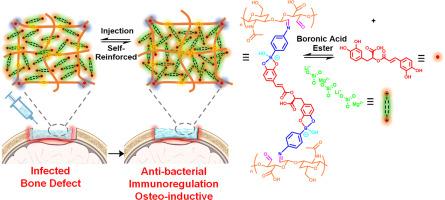
一种多功能自强化可注射水凝胶,可同时靶向巨噬细胞、细菌和骨髓基质细胞,加强感染性骨缺损的修复。
可注射水凝胶(IHs)在促进感染性骨缺损(IBDs)修复方面已显示出巨大潜力,但如何赋予其所需的抗菌、免疫调节和骨诱导特性并避免其在操作过程中出现机械故障仍是一项挑战。为此,我们开发了一种用于 IBD 修复的多功能 AOHA-RA/Lap 纳米复合材料 IH,它主要通过两种可逆交联构建而成:(这种纳米复合材料主要通过两种可逆交联构建:(i) 由青金石(Lap)晶体介导的静电相互作用;(ii) 4-氨基苯硼酸接枝氧化透明质酸(AOHA)和迷迭香酸(RA)之间的苯硼酸伊斯特键。由于其特殊的结构组成,AOHA-RA/Lap IH 被注射到骨缺损部位后,表现出优异的可注射性、自恢复性、空间适应性和自强化机械性能。此外,根据 Weibull 模型,RA 分子可从水凝胶中局部释放 10 天以上。系统的体外/体内试验证明,水凝胶对金黄色葡萄球菌(S. aureus)和大肠杆菌(E. coli)具有很强的抗菌活性。此外,还验证了水凝胶诱导巨噬细胞(Mφ)M2极化和骨髓基质细胞(BMSCs)成骨分化的能力,并确定前者的机制与JAK1-STAT1和PI3K-AKT信号通路有关,后者的机制与钙信号通路、细胞外基质(ECM)受体相互作用和TGF-β信号通路有关。将 AOHA-RA/Lap IH 植入金黄色葡萄球菌感染的大鼠颅骨缺损模型后,可显著加快 IBD 的修复,且不会引起明显的全身毒性。意义说明:利用迷迭香酸和青金石开发出一种可注射的水凝胶,有望在临床上加速修复感染性骨缺损。水凝胶的凝胶化完全由两种可逆交联驱动,这赋予了水凝胶卓越的空间适应性、自我恢复性和结构稳定性。制备的水凝胶具有优异的抗菌/抗生物膜活性,并能诱导巨噬细胞的 M2 极化和 BMSCs 的成骨分化。巨噬细胞极化的机制与 JAK1-STAT1 和 PI3K-AKT 信号通路有关。BMSCs 成骨分化的机制与 ECM 受体相互作用和钙信号/TGF-β 信号通路有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


