A multifunctional nanosystem catalyzed by cascading natural glucose oxidase and Fe3O4 nanozymes for synergistic chemodynamic and photodynamic cancer therapy
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The significance of the tumor microenvironment (TME) in tumor initiation and progression is increasingly acknowledged. Conventional therapeutic approaches face limitations within the complex TME, including the restrictions imposed by hypoxia on photodynamic therapy (PDT) and the deficiency of endogenous H₂O₂ affecting chemodynamic therapy (CDT). In response to the TME's characteristics of high metabolism, hypoxia, and weak acidity, a multifunctional nanosystem MNPs/GOD@CS/IR820, which synergistically integrates CDT and PDT, has been developed. This system can actively accumulate at tumor sites under an external magnetic field and release active components in response to the weakly acidic TME. It mitigates the limitations imposed by hypoxia and endogenous H₂O₂ deficiency on PDT and CDT, respectively, thereby enabling synergistic treatment. Additionally, the system's multimodal imaging capabilities facilitate precise tumor localization and real-time, non-invasive in vivo assessment via fluorescence imaging and MRI. In vitro and in vivo evaluations demonstrate significant antitumor efficacy, effectively inhibiting tumor growth and improving survival rates. By comprehensively addressing the challenges posed by the complex TME and enhancing real-time monitoring capabilities, our nanosystem paves the way for personalized and precise cancer treatment.
Statement of significance
This study introduces an innovative MNPs/GOD@CS/IR820 nanosystem that represents a significant advancement in cancer nanomedicine by addressing critical limitations of conventional photodynamic therapy (PDT), particularly in hypoxic tumor microenvironments. By synergistically integrating chemodynamic therapy (CDT) with PDT and incorporating MRI and fluorescence dual-modal imaging capabilities, this multifunctional platform offers enhanced therapeutic efficacy and real-time monitoring. The system's ability to generate oxygen in situ overcomes hypoxia-induced limitations, while its multimodal mechanism of action induces tumor cell apoptosis through multiple pathways. In vitro and in vivo studies demonstrate remarkable antitumor efficacy across diverse cancer types, significantly inhibiting tumor growth and improving survival rates. This comprehensive approach to cancer diagnosis and treatment not only advances precision medicine for targeted, multimodal cancer management but also provides a promising foundation for future clinical applications, potentially transforming cancer treatment strategies and improving patient outcomes.
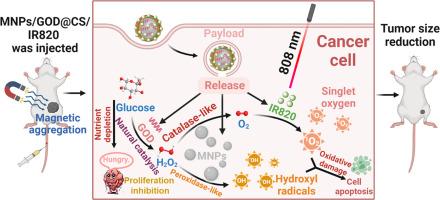
由级联天然葡萄糖氧化酶和 Fe3O4 纳米酶催化的多功能纳米生态系统,用于协同化学动力和光动力癌症治疗。
肿瘤微环境(TME)在肿瘤发生和发展过程中的重要性日益得到认可。传统的治疗方法在复杂的肿瘤微环境中受到限制,包括缺氧对光动力疗法(PDT)的限制,以及内源性 H₂O₂的缺乏对化学动力疗法(CDT)的影响。针对 TME 的高代谢、缺氧和弱酸性等特点,我们开发了一种多功能纳米系统 MNPs/GOD@CS/IR820,它能协同整合 CDT 和 PDT。该系统可在外部磁场作用下在肿瘤部位主动积聚,并在弱酸性 TME 作用下释放活性成分。它能缓解缺氧和内源性 H₂O₂ 缺乏分别对 PDT 和 CDT 造成的限制,从而实现协同治疗。此外,该系统的多模态成像功能有助于通过荧光成像和核磁共振成像进行精确的肿瘤定位和实时、无创的体内评估。体外和体内评估结果表明,该系统具有显著的抗肿瘤疗效,能有效抑制肿瘤生长并提高存活率。我们的纳米系统全面应对了复杂的 TME 带来的挑战,并增强了实时监测能力,为个性化和精确的癌症治疗铺平了道路。意义声明:本研究介绍了一种创新的 MNPs/GOD@CS/IR820 纳米系统,它解决了传统光动力疗法(PDT)的关键局限性,尤其是在缺氧的肿瘤微环境中,代表了癌症纳米医学的重大进展。通过将化学动力疗法(CDT)与光动力疗法协同整合,并结合核磁共振成像(MRI)和荧光双模态成像功能,该多功能平台提高了疗效和实时监测能力。该系统原位产生氧气的能力克服了缺氧引起的局限性,而其多模式作用机制可通过多种途径诱导肿瘤细胞凋亡。体外和体内研究表明,它对不同类型的癌症都有显著的抗肿瘤效果,能明显抑制肿瘤生长,提高生存率。这种全面的癌症诊断和治疗方法不仅推动了精准医学在癌症靶向、多模式治疗方面的发展,还为未来的临床应用奠定了良好的基础,有可能改变癌症治疗策略,改善患者预后。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
文献相关原料
公司名称
产品信息
阿拉丁
Ammonia in water
阿拉丁
1-ethyl-(3-dimethylaminopropyl) carbon diimide hydrochloride (EDC)
阿拉丁
N-hydroxysuccinimide (NHS)
阿拉丁
ferric chloride
阿拉丁
ferrous chloride
阿拉丁
3,3′5,5′-tetramethylbenzidine (TMB)
阿拉丁
anhydrous citric acid
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


