A convolutional attention model for predicting response to chemo-immunotherapy from ultrasound elastography in mouse tumor models
IF 5.4
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
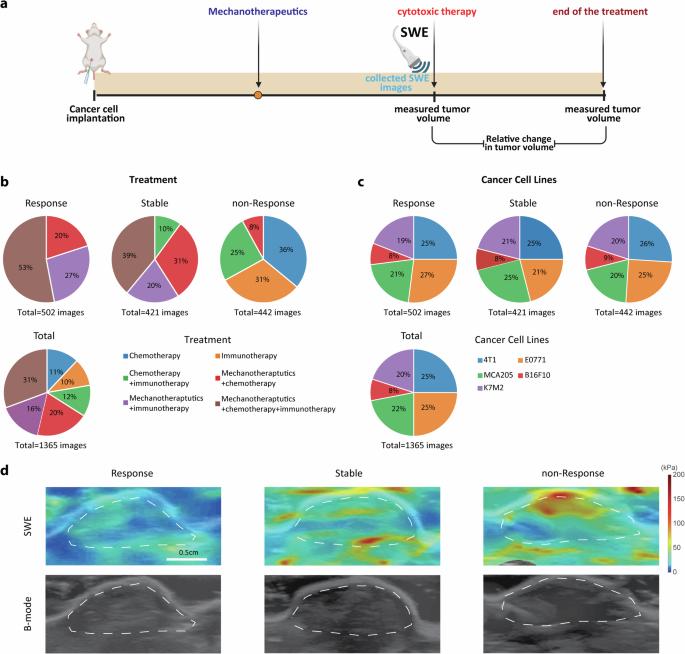
In the era of personalized cancer treatment, understanding the intrinsic heterogeneity of tumors is crucial. Despite some patients responding favorably to a particular treatment, others may not benefit, leading to the varied efficacy observed in standard therapies. This study focuses on the prediction of tumor response to chemo-immunotherapy, exploring the potential of tumor mechanics and medical imaging as predictive biomarkers. We have extensively studied “desmoplastic” tumors, characterized by a dense and very stiff stroma, which presents a substantial challenge for treatment. The increased stiffness of such tumors can be restored through pharmacological intervention with mechanotherapeutics. We developed a deep learning methodology based on shear wave elastography (SWE) images, which involved a convolutional neural network (CNN) model enhanced with attention modules. The model was developed and evaluated as a predictive biomarker in the setting of detecting responsive, stable, and non-responsive tumors to chemotherapy, immunotherapy, or the combination, following mechanotherapeutics administration. A dataset of 1365 SWE images was obtained from 630 tumors from our previous experiments and used to train and successfully evaluate our methodology. SWE in combination with deep learning models, has demonstrated promising results in disease diagnosis and tumor classification but their potential for predicting tumor response prior to therapy is not yet fully realized. We present strong evidence that integrating SWE-derived biomarkers with automatic tumor segmentation algorithms enables accurate tumor detection and prediction of therapeutic outcomes. This approach can enhance personalized cancer treatment by providing non-invasive, reliable predictions of therapeutic outcomes. Voutouri, Englezos et al. present a convolutional attention model utilizing ultrasound elastography for predicting chemo-immunotherapy responses in mouse tumors. Through training optimization on a large number of images, this approach highlights the potential of combining shear wave elastography with deep learning to enhance personalized cancer treatment. In personalized cancer treatment, it is important to understand that not all tumors respond the same way to therapy. While some patients may benefit from a particular treatment, others may not, leading to different outcomes. This study focuses on predicting how tumors will respond to a combination of chemotherapy and immunotherapy. Specifically, we looked at difficult-to-treat tumors with very stiff structures. These tumors can be softened with certain drugs making them more responsive to treatment. We developed a computer method to analyze medical images that measure the stiffness of tumors. Our method was trained on a large set of tumor images and was able to predict how well a tumor would respond to treatment. Overall, this approach could improve personalized cancer treatment using non-invasive medical imaging to predict which therapies will be most effective for each patient.
从小鼠肿瘤模型超声弹性成像预测化疗免疫疗法反应的卷积注意力模型。
背景:在个性化癌症治疗时代,了解肿瘤的内在异质性至关重要。尽管一些患者对某种治疗方法反应良好,但另一些患者可能无法从中获益,从而导致标准疗法的疗效参差不齐。这项研究的重点是预测肿瘤对化疗免疫疗法的反应,探索肿瘤力学和医学成像作为预测性生物标志物的潜力。我们对 "去瘤细胞 "肿瘤进行了广泛研究,这种肿瘤的特点是基质致密且非常僵硬,给治疗带来了巨大挑战。通过机械治疗药物的药理干预,可以恢复此类肿瘤增加的硬度:方法:我们开发了一种基于剪切波弹性成像(SWE)图像的深度学习方法,该方法涉及一个用注意力模块增强的卷积神经网络(CNN)模型。该模型被开发并评估为一种预测性生物标记物,用于检测肿瘤对化疗、免疫疗法或机械疗法联合用药后的反应性、稳定性和非反应性。我们从之前的实验中获得了 630 个肿瘤的 1365 幅 SWE 图像数据集,并将其用于训练和成功评估我们的方法。SWE 与深度学习模型相结合,在疾病诊断和肿瘤分类方面取得了可喜的成果,但其在治疗前预测肿瘤反应方面的潜力尚未得到充分发挥:我们提出了强有力的证据,证明将 SWE 衍生的生物标记物与自动肿瘤分割算法相结合可实现准确的肿瘤检测和治疗效果预测:结论:这种方法可以通过提供非侵入性、可靠的疗效预测来加强个性化癌症治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


