Bioenergetic-active exosomes for cartilage regeneration and homeostasis maintenance
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
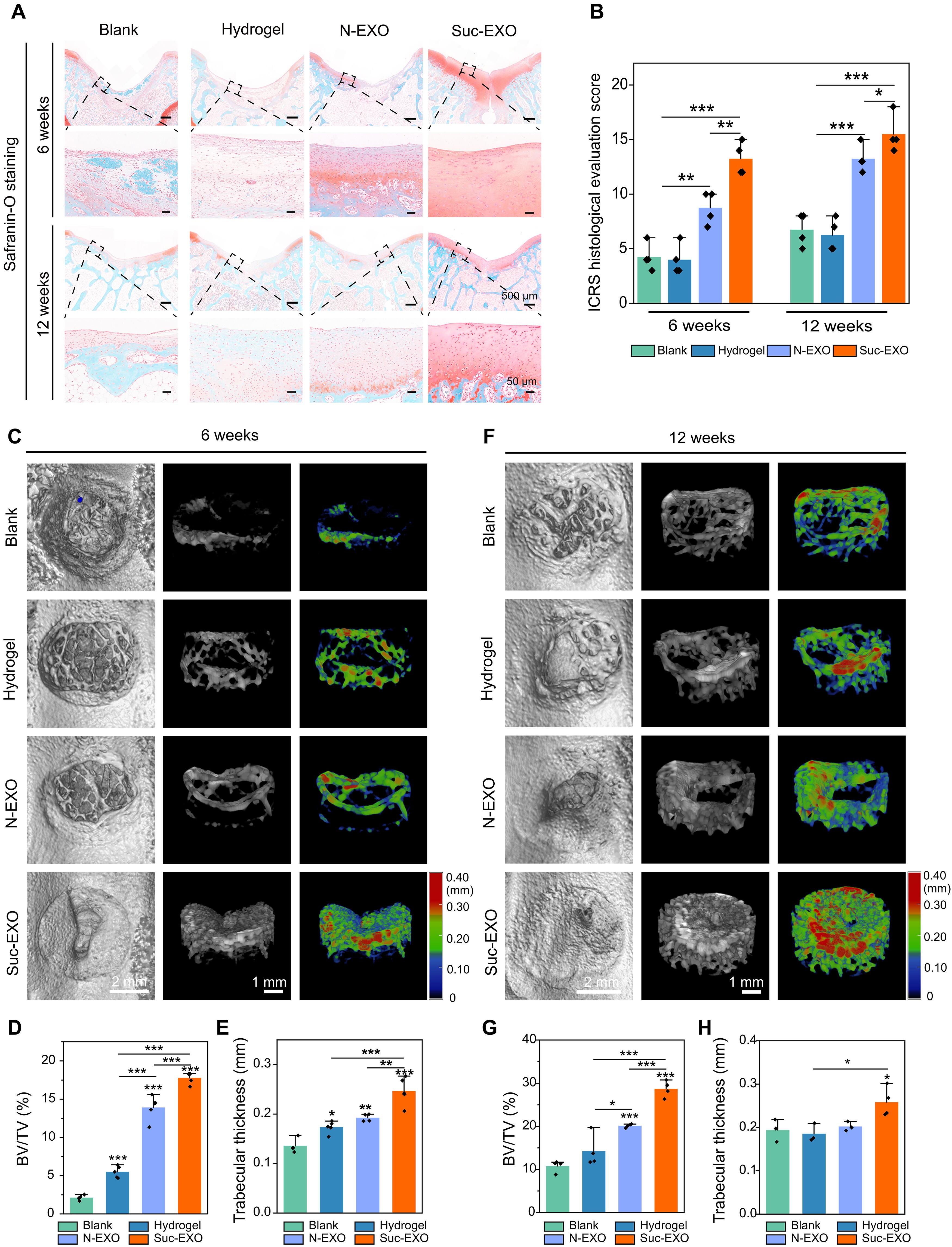
Cartilage regeneration relies on adequate and continuous bioenergy supply to facilitate cellular differentiation and extracellular matrix synthesis. Chondrocytes frequently undergo energy stress under pathological conditions, characterized by disrupted cellular metabolism and reduced adenosine triphosphate (ATP) levels. However, there has limited progress in modulating energy metabolism for cartilage regeneration thus far. Here, we developed bioenergetic-active exosomes (Suc-EXO) to promote cartilage regeneration and homeostasis maintenance. Suc-EXO exhibited a 5.42-fold increase in ATP content, enabling the manipulation of cellular energy metabolism by fueling the TCA cycle. With continuous energy supply, Suc-EXO promoted BMSC chondrogenic differentiation via the P2X7-mediated PI3K-AKT pathway. Moreover, Suc-EXO improved chondrocyte anabolism and mitochondrial homeostasis via the P2X7-mediated SIRT3 pathway. In a rabbit cartilage defect model, the Suc-EXO–encapsulated hydrogel notably promoted cartilage regeneration and maintained neocartilage homeostasis, leading to 2.26 and 1.53 times increase in Col2 and ACAN abundance, respectively. These findings make a remarkable breakthrough in modulating energy metabolism for cartilage regeneration, offering immense potential for clinical translation.
用于软骨再生和维持平衡的生物能活性外泌体
软骨再生依赖于充足而持续的生物能供应,以促进细胞分化和细胞外基质合成。在病理条件下,软骨细胞经常承受能量压力,其特点是细胞代谢紊乱和三磷酸腺苷(ATP)水平降低。然而,迄今为止,在调节能量代谢促进软骨再生方面取得的进展有限。在此,我们开发了生物能量活性外泌体(Suc-EXO),以促进软骨再生和维持体内平衡。Suc-EXO 的 ATP 含量增加了 5.42 倍,通过促进 TCA 循环实现了对细胞能量代谢的控制。在持续的能量供应下,Suc-EXO 通过 P2X7 介导的 PI3K-AKT 通路促进了 BMSC 软骨分化。此外,Suc-EXO 还能通过 P2X7 介导的 SIRT3 通路改善软骨细胞的新陈代谢和线粒体稳态。在兔软骨缺损模型中,Suc-EXO包裹的水凝胶显著促进了软骨再生并维持了新软骨的稳态,使Col2和ACAN的丰度分别增加了2.26倍和1.53倍。这些发现在调节能量代谢促进软骨再生方面取得了重大突破,为临床转化提供了巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
文献相关原料
公司名称
产品信息
麦克林
2% phosphotungstic acid
阿拉丁
NaIO4
阿拉丁
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
阿拉丁
1-hydroxybenzotriazole
阿拉丁
carbon hydrazide (CDH)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


