Benzofurans and dibenzofurans from galls on twigs of the endangered Chinese endemic tree Parrotia subaequalis and their inhibitory properties against Staphylococcus aureus and ATP-citrate lyase
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Parrotia subaequalis, an endangered Tertiary relict tree native to China and a member of the Hamamelidaceae family, is one of several host plant species in this family that exhibit unique ecological habits, such as gall formation. Tree galls are the results of complex interactions between gall-inducing insects and their host plant organs. The formation of galls may serve to protect other regions of the plant from potential damage, often through the production of phytoalexins. In this study, a preliminary investigation was carried out on the metabolites of the 90% MeOH extract derived from the closed spherical galls on the twigs of P. subaequalis. Consequently, nine previously undescribed benzofuran-type and dibenzofuran-type phytoalexins (parrotiagallols A−I, 1−9, respectively) were isolated and characterized, along with several known miscellaneous metabolites (10−17). Their chemical structures and absolute configurations were elucidated using spectroscopic methods, a combination of calculated and experimental electronic circular dichroism data, and single crystal X-ray diffraction analyses. Among these compounds, 1 and 2 are identified as neolignan derivatives, while compounds 3−5 are classified as 9,10-dinorneolignans. Compound 6 represents a rare 2,3-seco-neolignan, and compounds 7−9 are dihydroxy-dimethyl-dibenzofuran derivatives. Parrotiagallol A (1) showed considerable antibacterial activity against Staphylococcus aureus, with an MIC value of 14 μM. Additionally, parrotiagallol E (5) and methyl gallate (17) exhibited inhibitory effects against ATP-citrate lyase (ACL), a potential therapeutic target for hyperlipidemia, with IC50 values of 5.1 and 9.8 μM, respectively. The findings underscore that galls not only serve as physical defense barriers but also benefit from the chemical defense system of the host plants. These insights provide avenues for exploring potential new therapeutic agents for S. aureus infections and ACL-related diseases, while also promoting scientific conservation strategies for P. subaequalis.
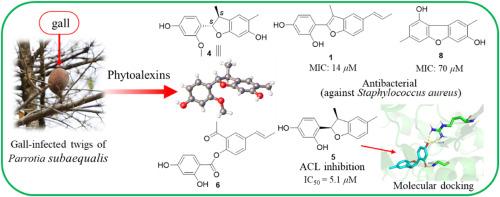
中国濒危特有树种亚桔梗虫瘿中的苯并呋喃和二苯并呋喃及其对金黄色葡萄球菌和 ATP-柠檬酸裂解酶的抑制性。
Parrotia subaequalis 是一种原产于中国的濒危第三纪孑遗树种,属于金缕梅科,是该科中表现出独特生态习性(如形成虫瘿)的几种寄主植物之一。树瘿是致瘿昆虫与其寄主植物器官之间复杂相互作用的结果。虫瘿的形成可能起到保护植物其他区域免受潜在损害的作用,通常是通过产生植物毒素。在本研究中,我们对从亚桔梗小枝上闭合球形虫瘿中提取的 90% MeOH 提取物中的代谢物进行了初步调查。结果分离并鉴定了九种以前未曾描述过的苯并呋喃类和二苯并呋喃类植物醛毒素(parrotiagallols A-I,分别为 1-9),以及几种已知的杂类代谢物(10-17)。利用光谱方法、计算和实验电子圆二色性数据组合以及单晶 X 射线衍射分析,阐明了这些化合物的化学结构和绝对构型。在这些化合物中,1 和 2 被确定为新木犀草素衍生物,而化合物 3-5 则被归类为 9,10-二龙脑木犀草素。化合物 6 是一种罕见的 2,3-seco-新木质素,而化合物 7-9 则是二羟基-二甲基-二苯并呋喃衍生物。Parrotiagallol A(1)对金黄色葡萄球菌具有相当强的抗菌活性,其 MIC 值为 14 μM。此外,Parrotiagallol E(5)和没食子酸甲酯(17)对高脂血症的潜在治疗靶点--ATP-柠檬酸裂解酶(ACL)具有抑制作用,其 IC50 值分别为 5.1 和 9.8 μM。这些发现强调,虫瘿不仅是物理防御屏障,还能从寄主植物的化学防御系统中获益。这些发现为探索金黄色葡萄球菌感染和前交叉韧带相关疾病的潜在新治疗药物提供了途径,同时也促进了亚桔梗的科学保护策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


