Antimicrobial peptide LyeTx I mn∆K labeled with 68Ga is a potential PET radiopharmaceutical for molecular imaging of infections
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
Abstract
Background
Antimicrobial peptides have been radiolabeled and investigated as molecular diagnostic probes due to their propensity to accumulate in infectious sites rather than aseptic inflammatory lesions. LyeTx I is a cationic peptide from the venom of Lycosa erythrognatha, exhibiting significant antimicrobial activity. LyeTx I mn∆K is a shortened derivative of LyeTx I, with an optimized balance between antimicrobial and hemolytic activities. This study reports the first 68Ga-radiolabeling of the DOTA-modified LyeTx I mn∆K and primarily preclinical evaluations of [68Ga]Ga-DOTA(K)-LyeTx I mn∆K as a PET radiopharmaceutical for infection imaging.
Methods
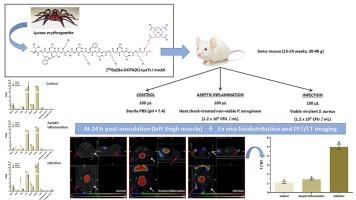
DOTA(K)-LyeTx I mn∆K was radiolabeled with freshly eluted 68Ga. Radiochemical yield (RCY), radiochemical purity (RCP), and radiochemical stability (in saline and serum) were evaluated using ascending thin-layer chromatography (TLC) and reversed-phase high-performance liquid chromatography (RP-HPLC). The radiopeptide's lipophilicity was assessed by determining the logarithm of the partition coefficient (Log P). Serum protein binding (SBP) and binding to Staphylococcus aureus (S. aureus) cells were determined in vitro. Ex vivo biodistribution studies and PET/CT imaging were conducted in healthy mice (control) and mice with infection and aseptic inflammation to evaluate the potential of [68Ga]Ga-DOTA(K)-LyeTx I mn∆K as a specific PET radiopharmaceutical for infections.
Results
[68Ga]Ga-DOTA(K)-LyeTx I mn∆K was obtained with a high RCY (>90 %), and after purification through a Sep-Pak C18 cartridge, the RCP exceeded 99 %. Ascending TLC and RP-HPLC showed that the radiopeptide remained stable for up to 3.0 h in saline solution and up to 1.5 h in murine serum. [68Ga]Ga-DOTA(K)-LyeTx I mn∆K exhibited hydrophilic characteristics (Log P = −2.4 ± 0.1) and low SPB (ranging from 23.3 ± 0.4 % at 5 min of incubation to 10.5 ± 1.1 % at 60 min of incubation). The binding of [68Ga]Ga-DOTA(K)-LyeTx I mn∆K to S. aureus cells was proportional to bacterial concentration, with binding percentages of 8.8 ± 0.5 % (0.5 × 109 CFU.mL−1), 16.2 ± 1.4 % (1.0 × 109 CFU.mL−1), and 62.2 ± 0.6 % (5.0 × 109 CFU.mL−1). Ex vivo biodistribution studies and PET/CT images showed higher radiopeptide uptake at the infection site compared to the aseptic inflammation site; the latter was similar to the control group. Target-to-non-target (T/NT) ratios obtained by ex vivo biodistribution data were approximately 1.0, 1.3, and 3.0 at all investigated time intervals for the control, aseptic inflammation, and infection groups, respectively. Furthermore, T/NT ratios obtained from PET/CT images were 1.1 ± 0.1 for the control group and 1.4 ± 0.1 for the aseptic inflammation group. For the infection group, T/NT ratio was 5.0 ± 0.3, approximately 5 times greater compared to the former groups.
Conclusions
The results suggest the potential of [68Ga]Ga-DOTA(K)-LyeTx I mn∆K as a PET radiopharmaceutical for molecular imaging of infections.
用 68Ga 标记的抗菌肽 LyeTx I mn∆K 是一种潜在的 PET 放射性药物,可用于感染的分子成像。
背景:由于抗菌肽具有在感染部位而非无菌性炎症病灶中蓄积的倾向,因此已将其作为分子诊断探针进行放射性标记和研究。LyeTx I 是一种阳离子肽,来自红腹狼毒,具有显著的抗菌活性。LyeTx I mn∆K 是 LyeTx I 的一种缩短衍生物,在抗菌和溶血活性之间取得了最佳平衡。本研究首次报道了对DOTA修饰的LyeTx I mn∆K进行68Ga放射性标记,并主要对[68Ga]Ga-DOTA(K)-LyeTx I mn∆K作为用于感染成像的PET放射性药物进行了临床前评估:用新鲜洗脱的 68Ga 对 DOTA(K)-LyeTx I mn∆K 进行放射性标记。使用上升薄层色谱法(TLC)和反相高效液相色谱法(RP-HPLC)评估了放射化学收率(RCY)、放射化学纯度(RCP)和放射化学稳定性(在生理盐水和血清中)。通过测定分配系数的对数(Log P)来评估放射肽的亲脂性。体外测定了血清蛋白结合力(SBP)和与金黄色葡萄球菌(S. aureus)细胞的结合力。在健康小鼠(对照组)和感染及无菌性炎症小鼠中进行了体内外生物分布研究和 PET/CT 成像,以评估[68Ga]Ga-DOTA(K)-LyeTx I mn∆K 作为感染特异性 PET 放射性药物的潜力:结果:获得的[68Ga]Ga-DOTA(K)-LyeTx I mn∆K RCY 高(>90%),经 Sep-Pak C18 滤芯纯化后,RCP 超过 99%。上升 TLC 和 RP-HPLC 显示,该放射肽在生理盐水中可保持稳定长达 3.0 小时,在小鼠血清中可保持稳定长达 1.5 小时。[68Ga]Ga-DOTA(K)-LyeTx I mn∆K 具有亲水性(Log P = -2.4 ± 0.1)和低 SPB(从孵育 5 分钟时的 23.3 ± 0.4 % 到孵育 60 分钟时的 10.5 ± 1.1 %)。68Ga]Ga-DOTA(K)-LyeTx I mn∆K 与金黄色葡萄球菌细胞的结合率与细菌浓度成正比,结合率分别为 8.8 ± 0.5 %(0.5 × 109 CFU.mL-1)、16.2 ± 1.4 %(1.0 × 109 CFU.mL-1)和 62.2 ± 0.6 %(5.0 × 109 CFU.mL-1)。体内外生物分布研究和 PET/CT 图像显示,感染部位的放射肽摄取量高于无菌性炎症部位;后者与对照组相似。体内外生物分布数据显示,对照组、无菌性炎症组和感染组在所有研究时间间隔内的目标与非目标(T/NT)比值分别约为 1.0、1.3 和 3.0。此外,通过 PET/CT 图像获得的 T/NT 比率在对照组为 1.1 ± 0.1,在无菌性炎症组为 1.4 ± 0.1。感染组的T/NT比值为5.0 ± 0.3,约为对照组的5倍:结果表明,[68Ga]Ga-DOTA(K)-LyeTx I mn∆K 有可能作为 PET 放射性药物用于感染的分子成像。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


