Hydrogen sulfide ameliorated endothelial dysfunction in hyperhomocysteinemia rats: Mechanism of IRE1α/JNK pathway-mediated autophagy
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
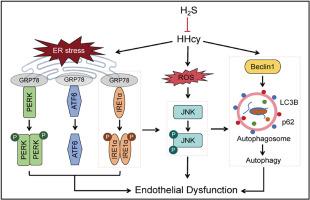
Previous studies showed that hyperhomocysteinemia (HHcy) induced endothelial dysfunction by endoplasmic reticulum (ER) stress induction and autophagy stimulation. This study aimed to determine the effect of hydrogen sulfide (H2S) in homocysteine (Hcy)-induced endothelial dysfunction and observe the possible mechanism involved. Male Wistar rats (160–180g) were used and randomly divided into four groups: Control group, HHcy group, HHcy+Sodium hydrosulfide (NaHS) group and NaHS group. Rats were fed with 2% high methionine diet for 8 weeks to set up HHcy model. Plasma concentration of Hcy was measured by ELISA. Endothelium-dependent and non-endothelium-dependent vasodilation of rat renal arteries were determined by myograph. The protein expression of cystathionine-γ-lyase (CSE), ER stress- and autophagy-related proteins in renal arteries or human umbilical vein endothelial cells (HUVECs) were analyzed by western blotting. The endothelial function was impaired in HHcy rats and HUVECs. NaHS supplementation could improve the ACh-induced vasodilation, however it was eliminated by ER stress inducer Tunicamycin (TM) or autophagy inducer Rapamycin. Western blotting in renal arteries showed that Glucose-regulated protein 78 (GRP78) and three branches of ER stress (p-IRE1α, p-PERK, ATF6) , p-JNK1+p-JNK2 were downregulated, simultaneously the autophagy marker Beclin1, LC3BII/LC3BI ratio were decreased and p62 was increased with NaHS treatment in HHcy rats. In HUVECs, IRE1α-JNK induced autophagy was involved in HHcy-induced endothelial dysfunction, while NaHS stimulation reversed the protein expression in IRE1α/JNK-autophagy pathway with Hcy incubation. This study might suggest that endothelial dysfunction induced by HHcy might be correlated with IRE1α-JNK-autophagy axis pathway, which was suppressed by exogenous supplementation of H2S donor, NaHS.
硫化氢可改善高同型半胱氨酸血症大鼠的内皮功能障碍:IRE1α/JNK通路介导的自噬机制
先前的研究表明,高同型半胱氨酸血症(HHcy)通过内质网(ER)应激诱导和自噬刺激诱导内皮功能障碍。本研究旨在确定硫化氢(H2S)对同型半胱氨酸(Hcy)诱导的内皮功能障碍的影响,并观察其可能的机制。采用雄性 Wistar 大鼠(160-180 克),随机分为四组:对照组、HHcy 组、HHcy+硫氢化钠(NaHS)组和 NaHS 组。用 2% 高蛋氨酸饮食喂养大鼠 8 周,建立 HHcy 模型。用 ELISA 法检测血浆中 Hcy 的浓度。用肌电图测定大鼠肾动脉的内皮依赖性和非内皮依赖性血管扩张。蛋白印迹法分析了肾动脉或人脐静脉内皮细胞(HUVECs)中胱硫醚-γ-裂解酶(CSE)、ER应激和自噬相关蛋白的表达。结果表明,HHcy 大鼠和 HUVECs 的内皮功能受损。补充 NaHS 可改善 ACh 诱导的血管扩张,但 ER 应激诱导剂 Tunicamycin(TM)或自噬诱导剂雷帕霉素可消除这种扩张。肾动脉中的 Western 印迹显示,NaHS 处理 HHcy 大鼠后,葡萄糖调节蛋白 78(GRP78)和ER 应激的三个分支(p-IRE1α、p-PERK、ATF6)、p-JNK1+p-JNK2 下调,同时自噬标志物 Beclin1、LC3BII/LC3BI 比值降低,p62 升高。在HUVECs中,IRE1α-JNK诱导的自噬参与了HHcy诱导的内皮功能障碍,而NaHS刺激可逆转Hcy孵育下IRE1α/JNK-自噬通路的蛋白表达。这项研究可能表明,HHcy诱导的内皮功能障碍可能与IRE1α-JNK-自噬轴通路有关,而外源性补充H2S供体NaHS可抑制该通路的表达。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nitric oxide : biology and chemistry
生物-生化与分子生物学
CiteScore
7.50
自引率
7.70%
发文量
74
审稿时长
52 days
期刊介绍:
Nitric Oxide includes original research, methodology papers and reviews relating to nitric oxide and other gasotransmitters such as hydrogen sulfide and carbon monoxide. Special emphasis is placed on the biological chemistry, physiology, pharmacology, enzymology and pathological significance of these molecules in human health and disease. The journal also accepts manuscripts relating to plant and microbial studies involving these molecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


