Choroidal melanocyte secretome from cultured cells and tissue-engineered choroid models exposed to acute or chronic oxidative stress
IF 3
2区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
Abstract
The choroid, located between the retina and the sclera, is a vascularized and pigmented connective tissue, playing a crucial role in providing oxygen and nutrients to the outer layers of the retina, and in absorbing excessive light. How choroidal melanocytes (CMs) participate in tissue homeostasis through paracrine signaling with neighboring cells is poorly understood. In this study, using two-dimensional and three-dimensional models, we aimed to identify proteins secreted by CMs under different oxidative stress conditions. To do so, CMs, choroidal fibroblasts (CFs), and retinal pigment epithelial (RPE) cells were isolated from native human RPE/choroidal tissues and expanded. RNA was isolated and processed for gene profiling analysis. The self-assembly approach of tissue engineering was used to form 3D stromal substitutes, and RPE cells and/or CMs were added to produce 3D models with different cell combinations. The medium conditioned by cells in 2D and 3D cultures was collected in a non-stressed condition and following acute or chronic oxidative stress exposures, then proteome and ELISA analyses were performed to identify cytokines secreted majorly by CMs. RNA analysis revealed 15 secretome-related transcripts that were more abundantly expressed in CMs compared to the other 2 cell types, including serpin family F member 1 (SERPINF1) (coding for pigment epithelium-derived factor; PEDF) and secreted phosphoprotein 1 (SPP1) (coding for osteopontin). At the protein level, the expression of osteopontin and PEDF was higher in CMs of different age groups compared to CFs and RPE cells. In the 3D models containing CMs, cytokine arrays also identified macrophage inflammatory protein (MIP)-1α/MIP-1β in non-stressed, MIP-1α/MIP-1β, interleukin (IL)-24, and angiogenin following an acute oxidative stress, and macrophage migration inhibitory factor (MIF), granulocyte-colony stimulating factor (G-CSF), intercellular adhesion molecule-1 (ICAM-1), and IL-1α following a chronic oxidative stress. This study identifies for the first time trophic factors secreted by CMs that could influence neighboring cells through paracrine signaling. Of those, PEDF and osteopontin are antioxidative proteins that are known to attenuate oxidative stress damage. Identifying factors that can help manage oxidative stress in the posterior segment of the eye may lead to promising treatments for retinal diseases.
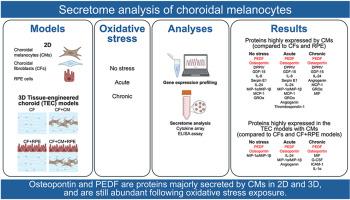
来自暴露于急性或慢性氧化应激的培养细胞和组织工程脉络膜模型的脉络膜黑色素细胞分泌物。
脉络膜位于视网膜和巩膜之间,是一种血管和色素结缔组织,在为视网膜外层提供氧气和营养物质以及吸收过量光线方面起着至关重要的作用。人们对脉络膜黑色素细胞(CMs)如何通过与邻近细胞的旁分泌信号参与组织平衡还知之甚少。在这项研究中,我们利用二维和三维模型,旨在鉴定 CMs 在不同氧化应激条件下分泌的蛋白质。为此,我们从原生人类 RPE/脉络膜组织中分离并扩增了 CMs、脉络膜成纤维细胞(CFS)和视网膜色素上皮细胞(RPE)。对 RNA 进行了分离和处理,以进行基因谱分析。利用组织工程的自组装方法形成三维基质替代物,并加入 RPE 细胞和/或 CMs 以产生具有不同细胞组合的三维模型。收集二维和三维培养细胞在非应激状态下和急性或慢性氧化应激暴露后的培养基,然后进行蛋白质组和 ELISA 分析,以确定 CMs 主要分泌的细胞因子。RNA分析显示,与其他两种细胞类型相比,有15种分泌物相关转录本在CMs中表达更为丰富,其中包括丝氨酸蛋白家族F成员1(SERPINF1)(编码色素上皮衍生因子;PEDF)和分泌磷蛋白1(SPP1)(编码骨化素)。在蛋白质水平上,与 CFs 和 RPE 细胞相比,不同年龄组的 CMs 中骨质素和 PEDF 的表达量更高。在含有CMs的三维模型中,细胞因子阵列还发现了非应激状态下的巨噬细胞炎症蛋白(MIP)-1α/MIP-1β、急性氧化应激后的MIP-1α/MIP-1β、白细胞介素(IL)-24和血管生成素,以及慢性氧化应激后的巨噬细胞迁移抑制因子(MIF)、粒细胞集落刺激因子(G-CSF)、细胞间粘附分子-1(ICAM-1)和IL-1α。这项研究首次发现了中胚层组织分泌的营养因子,它们可通过旁分泌信号影响邻近细胞。其中,PEDF和Osteopontin是已知能减轻氧化应激损伤的抗氧化蛋白。找出有助于控制眼球后段氧化应激的因子,可能会为治疗视网膜疾病带来希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental eye research
医学-眼科学
CiteScore
6.80
自引率
5.90%
发文量
323
审稿时长
66 days
期刊介绍:
The primary goal of Experimental Eye Research is to publish original research papers on all aspects of experimental biology of the eye and ocular tissues that seek to define the mechanisms of normal function and/or disease. Studies of ocular tissues that encompass the disciplines of cell biology, developmental biology, genetics, molecular biology, physiology, biochemistry, biophysics, immunology or microbiology are most welcomed. Manuscripts that are purely clinical or in a surgical area of ophthalmology are not appropriate for submission to Experimental Eye Research and if received will be returned without review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


