RAD51 recombinase and its paralogs: Orchestrating homologous recombination and unforeseen functions in protozoan parasites
IF 1.4
4区 医学
Q3 PARASITOLOGY
引用次数: 0
Abstract
The DNA of protozoan parasites is highly susceptible to damage, either induced by environmental agents or spontaneously generated during cellular metabolism through reactive oxygen species (ROS). Certain phases of the cell cycle, such as meiotic recombination, and external factors like ionizing radiation (IR), ultraviolet light (UV), or chemical genotoxic agents further increase this susceptibility. Among the various types of DNA damage, double-stranded breaks (DSBs) are the most critical, as they are challenging to repair and can result in genetic instability or cell death. DSBs caused by environmental stressors are primarily repaired via one of two major pathways: non-homologous end joining (NHEJ) or homologous recombination (HR). In multicellular eukaryotes, NHEJ predominates, but in unicellular eukaryotes such as protozoan parasites, HR seems to be the principal mechanism for DSB repair. The HR pathway is orchestrated by proteins from the RAD52 epistasis group, including RAD51, RAD52, RAD54, RAD55, and the MRN complex. This review focuses on elucidating the diverse roles and significance of RAD51 recombinase and its paralogs in protozoan parasites, such as Acanthamoeba castellanii, Entamoeba histolytica (Amoebozoa), apicomplexan parasites (Chromalveolata), Naegleria fowleri, Giardia spp., Trichomonas vaginalis, and trypanosomatids (Excavata), where they primarily function in HR. Additionally, we analyze the diversity of proteins involved in HR, both upstream and downstream of RAD51, and discuss the implications of these processes in parasitic protozoa.
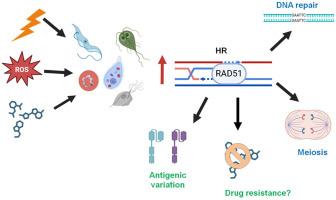
RAD51 重组酶及其旁系亲属:在原生动物寄生虫中协调同源重组和意外功能。
原生动物寄生虫的 DNA 极易受到损伤,这种损伤可能是环境因素诱发的,也可能是细胞代谢过程中通过活性氧(ROS)自发产生的。细胞周期的某些阶段,如减数分裂重组,以及电离辐射(IR)、紫外线(UV)或化学基因毒性物质等外部因素,都会进一步增加这种易感性。在各种类型的 DNA 损伤中,双链断裂(DSB)最为关键,因为它们难以修复,可能导致基因不稳定或细胞死亡。环境应激因素造成的 DSB 主要通过两种主要途径之一进行修复:非同源末端连接(NHEJ)或同源重组(HR)。在多细胞真核生物中,NHEJ占主导地位,但在原生动物寄生虫等单细胞真核生物中,HR似乎是DSB修复的主要机制。HR途径由RAD52外显子组的蛋白质协调,包括RAD51、RAD52、RAD54、RAD55和MRN复合体。本综述重点阐明了 RAD51 重组酶及其旁系亲属在原生动物寄生虫(如棘阿米巴、组织溶解恩塔米巴虫(阿米巴原虫)、阿米巴复合寄生虫(Chromalveolata)、福氏瑙格勒氏虫、贾第鞭毛虫属、阴道毛滴虫和锥虫(Excavata))中的不同作用和意义,它们在这些寄生虫中主要发挥 HR 功能。此外,我们还分析了 RAD51 上下游参与 HR 的蛋白质的多样性,并讨论了这些过程对寄生原生动物的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental parasitology
医学-寄生虫学
CiteScore
3.10
自引率
4.80%
发文量
160
审稿时长
3 months
期刊介绍:
Experimental Parasitology emphasizes modern approaches to parasitology, including molecular biology and immunology. The journal features original research papers on the physiological, metabolic, immunologic, biochemical, nutritional, and chemotherapeutic aspects of parasites and host-parasite relationships.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


