Simvastatin and adenosine-co-loaded nanostructured lipid carriers for wound healing: Development, characterization and cell-based investigation
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-15
DOI:10.1016/j.ejpb.2024.114533
引用次数: 0
Abstract
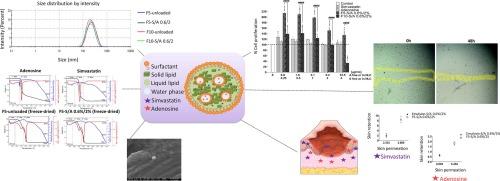
Chronic wounds represent a significant global health burden, characterized by delayed skin healing and associated comorbidities. The present study aimed to develop nanostructured lipid carriers (NLCs) as a topical delivery system for the co-administration of simvastatin and adenosine to address chronic wound management. The rationale behind the co-delivery approach was to mitigate the cytotoxicity associated with high-dose simvastatin, while preserving its therapeutic benefits through a potential synergistic or additive effect. A significant challenge in the development of these NLCs was the encapsulation of the highly hydrophilic adenosine within the hydrophobic lipid matrix. The NLCs were prepared using a hot homogenization-sonication method with a double emulsion technique and optimized through a series of formulation trials, employing various surfactants, solid and liquid lipids, to achieve efficient drug encapsulation, particularly for the hydrophilic adenosine. Optimized formulations F5- and F10-S/A 0.6 %/2 % (containing 0.6 % simvastatin and 2 % adenosine), exhibited promising physicochemical properties. The main difference was the liquid lipid used: F5 contained Miglyol 810 N, while F10 contained Capmul MCM C-8. Both formulations displayed a mean particle size below 230 nm, a polydispersity index (PDI) of approximately 0.2, and a zeta potential around –22 mV. While simvastatin association efficiency (AE) was nearly 100 %, adenosine AE was higher for F10 (24 %), compared to F5 (13.5 %). F5 demonstrated superior stability compared to F10, maintaining consistent particle size and PDI over a 60-day period. Formulation F5 also demonstrated superior cell-based in vitro performance compared to F10, with higher cell viability (MTT assay), greater cell proliferation induction (SRB assay), and enhanced cell proliferation and migration in the wound-scratch assay. While F10 displayed higher adenosine AE, F5 excelled in terms of stability and biological activity. The slight increase in intracellular reactive oxygen species levels observed with F5 may contribute to its enhanced proliferative effects. In-depth characterization revealed that F5 comprised spherical nanoparticles, and thermal analysis indicated no significant changes in the nanocarrier structure upon drug encapsulation. Additionally, ex vivo permeability study demonstrated superior skin retention of both simvastatin and adenosine for F5 compared to an emulsion control. Overall, the F5 nanocarrier demonstrated suitable physicochemical properties, cellular biocompatibility, induction of cell proliferation and migration events, and drug retention capacity in the skin layers, indicating its potential as a promising topical treatment for difficult-to-heal wounds.
用于伤口愈合的辛伐他汀和腺苷共负载纳米结构脂质载体:开发、表征和基于细胞的研究。
慢性伤口是一种严重的全球健康负担,其特点是皮肤愈合延迟和相关并发症。本研究旨在开发纳米结构脂质载体(NLC),作为联合给药辛伐他汀和腺苷的局部给药系统,以解决慢性伤口管理问题。联合给药方法的原理是减轻大剂量辛伐他汀的细胞毒性,同时通过潜在的协同或叠加效应保留其治疗功效。开发这种 NLCs 的一个重大挑战是如何在疏水性脂质基质中封装高亲水性腺苷。我们采用热均质化-声化方法和双乳液技术制备了 NLCs,并通过一系列配方试验进行了优化,采用了各种表面活性剂、固态和液态脂质,以实现药物的高效封装,尤其是对亲水性腺苷的封装。优化配方 F5- 和 F10-S/A 0.6 %/2%(含 0.6 % 辛伐他汀和 2 % 腺苷)显示出良好的理化特性。主要区别在于所使用的液态脂质:F5 含有 Miglyol 810 N,而 F10 则含有 Capmul MCM C-8。两种制剂的平均粒径均小于 230 nm,多分散指数(PDI)约为 0.2,zeta 电位约为 -22 mV。辛伐他汀的关联效率(AE)接近 100%,而 F10 的腺苷关联效率(AE)为 24%,高于 F5(13.5%)。F5 的稳定性优于 F10,在 60 天的时间内保持了一致的粒度和 PDI。与 F10 相比,制剂 F5 还具有更好的细胞体外性能,细胞存活率更高(MTT 试验),细胞增殖诱导能力更强(SRB 试验),在伤口划痕试验中细胞增殖和迁移能力更强。虽然 F10 显示出更高的腺苷 AE,但 F5 在稳定性和生物活性方面更胜一筹。观察到 F5 细胞内活性氧水平略有增加,这可能是其增殖效果增强的原因。深入表征显示,F5 由球形纳米颗粒组成,热分析表明药物封装后纳米载体结构无明显变化。此外,体内外渗透性研究表明,与乳液对照组相比,F5 对辛伐他汀和腺苷的皮肤保留率更高。总之,F5 纳米载体表现出了合适的理化特性、细胞生物相容性、诱导细胞增殖和迁移事件以及在皮肤层中的药物保留能力,这表明它有望成为一种治疗难以愈合伤口的局部疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


