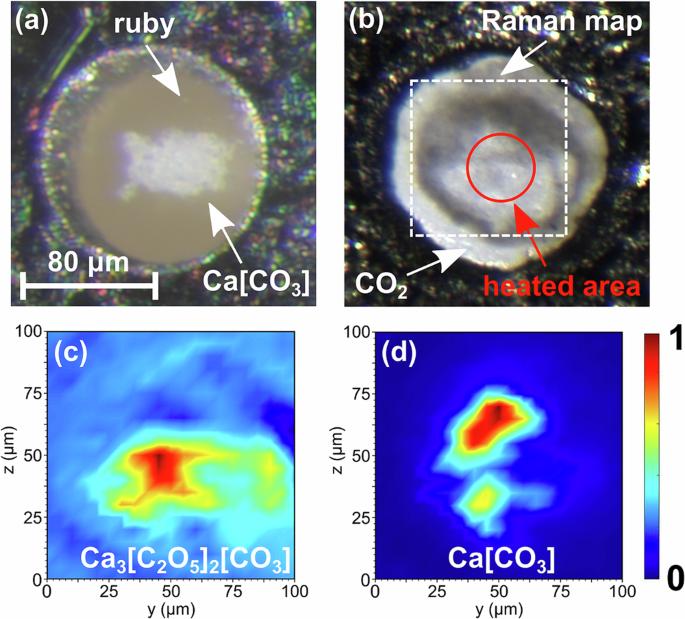
Ca3[C2O5]2[CO3] is a pyrocarbonate which can be formed at p, T-conditions prevalent in the Earth’s transition zone
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Understanding the fate of subducted carbonates is a prerequisite for the elucidation of the Earth’s deep carbon cycle. Here we show that the concomitant presence of Ca[CO3] with CO2 in a subducting slab very likely results in the formation of an anhydrous mixed pyrocarbonate, $${{{{\rm{Ca}}}}}_{3}{\left[{{{{\rm{C}}}}}_{2}{{{{\rm{O}}}}}_{5}\right]}_{2}\left[{{{{\rm{CO}}}}}_{3}\right]$$ , at moderate pressure ( ≈ 20 GPa) and temperature ( ≈ 1500 K) conditions. We show that at these conditions $${{{{\rm{Ca}}}}}_{3}{\left[{{{{\rm{C}}}}}_{2}{{{{\rm{O}}}}}_{5}\right]}_{2}\left[{{{{\rm{CO}}}}}_{3}\right]$$ can be obtained by reacting Ca[CO3] with CO2 in a laser-heated diamond anvil cell. The crystal structure was obtained from synchrotron-based single crystal X-ray diffraction data. Density Functional Perturbation Theory calculations in combination with experimental Raman spectroscopy results unambiguously confirmed the structural model. The crystal structure of $${{{{\rm{Ca}}}}}_{3}{\left[{{{{\rm{C}}}}}_{2}{{{{\rm{O}}}}}_{5}\right]}_{2}\left[{{{{\rm{CO}}}}}_{3}\right]$$ is characterized by the presence of $${\left[{{{{\rm{CO}}}}}_{3}\right]}^{2-}$$ - and $${\left[{{{{\rm{C}}}}}_{2}{{{{\rm{O}}}}}_{5}\right]}^{2-}$$ -groups. The results presented here imply that the formation of $${{{{\rm{Ca}}}}}_{3}{\left[{{{{\rm{C}}}}}_{2}{{{{\rm{O}}}}}_{5}\right]}_{2}\left[{{{{\rm{CO}}}}}_{3}\right]$$ needs to be taken into account when constructing models of the deep carbon cycle of the Earth. Carbonates are transported into the deep Earth by subduction of the oceanic lithosphere, but the stability fields of subducted carbonates as a function of pressure, temperature, and composition remain incompletely described. Here, the authors synthesize the anhydrous, mixed pyrocarbonate Ca3[C2O5]2[CO3] from Ca[CO3] and CO2 in a laser-heated diamond anvil cell at moderate pressure and elucidate its structural features.
Ca3[C2O5]2[CO3]是一种热碳酸盐,可在地球过渡带普遍存在的 p、T 条件下形成。
了解俯冲碳酸盐的命运是阐明地球深部碳循环的先决条件。在这里,我们展示了在中等压力(≈ 20 GPa)和温度(≈ 1500 K)条件下,Ca[CO3]与二氧化碳同时存在于俯冲板块中很可能会形成无水混合碳酸氢盐--Ca 3 C 2 O 5 2 CO 3。我们的研究表明,在这些条件下,通过在激光加热的金刚石砧槽中使 Ca[CO3] 与 CO2 反应,可以得到 Ca 3 C 2 O 5 2 CO 3。晶体结构是从同步辐射单晶 X 射线衍射数据中获得的。密度泛函扰动理论计算结合拉曼光谱实验结果明确证实了该结构模型。Ca 3 C 2 O 5 2 CO 3 晶体结构的特点是存在 CO 3 2 - 和 C 2 O 5 2 - 基团。本文介绍的结果表明,在构建地球深层碳循环模型时,需要考虑 Ca 3 C 2 O 5 2 CO 3 的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


