Retrospective analysis of rapid drug desensitization with biologic agents: A single center experience
Abstract
Background
Following the increased use of biological agents, a subset of patients experiences hypersensitivity reaction (HSR). We reported our experience with rapid drug desensitization (RDD) to nine biologics (rituximab, infliximab, cetuximab, trastuzumab, pertuzumab, nivolumab, brentuximab, tocilizumab and filgrastim) and identified risk factors for breakthrough reactions (BTRs).
Method
This was a retrospective review (2013–2022) of patients with immediate HSRs to biological agents. Initial HSRs were classified as grade 1, 2, or 3 in their severity. Skin prick tests (SPT)/intradermal tests (IDT) were performed using implicated agents. The phenotypes of HSRs were defined as Type I, cytokine-release syndrome (CRS), mixed reactions (cytokine-release + type I) based on history, clinical presentations and skin tests with implicated biologicals. A 12-step RDD protocol was used.
Results
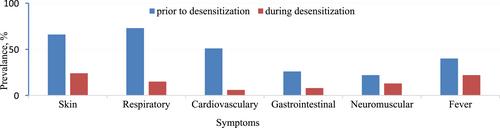
The study comprised 45 patients (F/M: 31/14, median age: 55 (range: 20–69)). Majority of the patients reacted at the first infusion (n: 29/45, 64.4%). The majority of initial HSRs were grade 3 (n: 24/45, 53.3%) and grade 2 (n: 21/45, 46.6%); none were grade 1. Initial reactions were presented as type I (n: 20/45, 44.4%), CRS (n: 12/45, 26.6%) and mixed (n: 13/45, 28.8%). A total of 258 RDDs were performed and 98.4% of them were completed successfully. BTRs occurred in 36/258 (13.9%) infusions of RDDs. There was no significant association between the BTRs and age, drug cycle, SPT and IDT positivity, gender, comorbidities, or atopy.
Conclusion
In our experience, 98.4% of 258 RDDs to biologics were successfully completed; RDD was safe and effective for our population.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


