Enriched environment enhances angiogenesis in ischemic stroke through SDF-1/CXCR4/AKT/mTOR pathway
IF 4.4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Environmental-gene interactions significantly influence various bodily functions. Enriched environment (EE), a non-pharmacological treatment method, enhances angiogenesis in ischemic stroke (IS). However, underlying the role of EE in angiogenesis in aged mice post-IS remain unclear. This study aimed to determine the potential mechanism by which EE mediates angiogenesis in 12-month-old IS mice and oxygen-glucose deprivation/reperfusion (OGD/R)-induced bEnd.3 cells. In vivo, EE treatment alleviated the neurological deficits, enhanced angiogenesis, upregulated SDF-1, VEGFA, and the AKT/mTOR pathway. In addition, exogenous SDF-1 treatment had a protective effect similar to that of EE treatment in aged mice with IS. However, SDF-1 neutralizing antibody, AMD3100 (CXCR4 inhibitor), ARQ092 (AKT inhibitor), and rapamycin (mTOR inhibitor) treatment blocked the neuroprotective effect of EE treatment and inhibited angiogenesis in IS mice. In vitro, exogenous SDF-1 promoted migration of OGD/R-induced bEnd.3 cells and activated the AKT/mTOR pathway. AMD3100, ARQ092, and rapamycin inhibited SDF-1-induced cell migration. Collectively, these findings demonstrate that EE enhances angiogenesis and improves the IS outcomes through SDF-1/CXCR4/AKT/mTOR pathway.
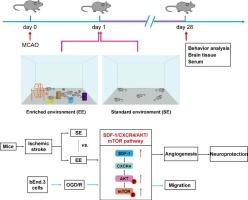
富集环境通过 SDF-1/CXCR4/AKT/mTOR 通路促进缺血性中风的血管生成
环境与基因之间的相互作用极大地影响着人体的各种功能。富集环境(EE)作为一种非药物治疗方法,可增强缺血性中风(IS)的血管生成。然而,EE在缺血性脑卒中后老年小鼠血管生成中的作用尚不清楚。本研究旨在确定EE介导12个月大的IS小鼠和氧-葡萄糖剥夺/再灌注(OGD/R)诱导的bEnd.3细胞血管生成的潜在机制。在体内,EE治疗缓解了神经功能缺损,增强了血管生成,上调了SDF-1、VEGFA和AKT/mTOR通路。此外,外源性SDF-1治疗对老年IS小鼠的保护作用与EE治疗相似。然而,SDF-1中和抗体、AMD3100(CXCR4抑制剂)、ARQ092(AKT抑制剂)和雷帕霉素(mTOR抑制剂)的治疗阻断了EE治疗对IS小鼠神经的保护作用,并抑制了血管生成。在体外,外源性 SDF-1 可促进 OGD/R 诱导的 bEnd.3 细胞迁移并激活 AKT/mTOR 通路。AMD3100、ARQ092和雷帕霉素抑制了SDF-1诱导的细胞迁移。总之,这些研究结果表明,EE可通过SDF-1/CXCR4/AKT/mTOR途径增强血管生成并改善IS的结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


