Early divergent modulation of NLRP2′s and NLRP3′s inflammasome sensors vs. AIM2′s one by signals from Aβ·Calcium-sensing receptor complexes in human astrocytes
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Alzheimer’s disease (AD), the most prevalent human dementia, is driven by accruals of extracellular Aβ42 senile patches and intracellular neurofibrillary tangles of hyperphosphorylated Tau (p-Tau) proteins. AD’s concurrent neuroinflammation is prompted by innate immunity-related cytosolic protein oligomers named inflammasomes. Upon proper “first” (priming) and “second” (activating) signals, inflammasomes overproduce proinflammatory Interleukin (IL)-1β, and IL-18 while cleaving pyroptosis-promoting Gasdermin D’s N-terminal fragments. Our earlier studies highlighted that in pure monocultures, exogenous Aβ25-35-treated nonproliferating human cortical astrocytes (HCAs) made and released surpluses of endogenous Aβ42-oligomers (−os) and p-Tau-os, just as alike-treated human cortical neurons did. Aβ25-35-exposed HCAs also over-released NO, VEGFA, and IL-6. Aβ•CaSR (Aβ·Calcium-Sensing Receptor) complexes generated intracellular signals mediating all such neurotoxic effects since CaSR’s negative allosteric modulators (aka NAMs or calcilytics, e.g., NPS2143) fully suppressed them. However, it had hitherto remained unexplored whether signals from Aβ·CaSR complexes also induced the early expression and/or activation of NOD-like 2 (NLRP2) and 3 (NLRP3) and of PYHIN absent in melanoma 2 (AIM2) inflammasomes in monocultured HCAs. To clarify this topic, we used in-situ-Proximity Ligation, qRT-PCR, double antibody arrays, immunoblots, and Caspase 1/4 enzymatic assays. Aβ·CaSR complexes quickly assembled on HCAs surface and issued intracellular signals activating Akt and JAK/STAT axes. In turn, the latter upregulated NLRP2 and NLRP3 PRRs (pattern recognition receptors) yet downregulated AIM2. These effects were specific, being significantly hindered by NPS2143 and inhibitors of PI3K (LY294002), AMPKα (Dorsomorphin), mTOR (Torin1), and JAK/TYK (Brepoticinib). A wide-spectrum inhibitor, Bay11-7082, intensified the Aβ·CaSR/Akt/JAK/STAT axis-driven opposite control of NLRP3’s and AIM2’s PRR proteins without affecting NLRP2 PRR upregulation. However, the said effects on the PRRs proteins vanished within 24-h. Moreover, Aβ·CaSR signals neither concurrently changed ASC, pro-IL-1β, and Gasdermin-D (holo- and fragments) protein levels and Caspases 1 and 4 enzymatic activities nor induced pyroptosis. Therefore, Aβ·CaSR cues acted as “first (priming) signals” temporarily increasing NLRP2 and NLRP3 PRRs expression without activating the corresponding inflammasomes. The neatly divergent modulation of NLRP3’s vs. AIM2’s PRR proteins by Aβ·CaSR cues and by Bay11-7082 suggests that, when bacterial or viral DNA fragments are absent, AIM2 might play “anti-inflammasomal” or other roles in HCAs. However, Bay11-7082’s no effect on NLRP2 PRR overexpression also reveals that CaSR’s downstream mechanisms controlling inflammasomes’ sensors are quite complex in HCAs, and hence, given AD’s impact on human health, well worth further studies.
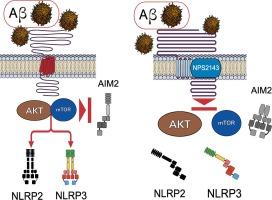
在人类星形胶质细胞中,来自 Aβ-钙传感受体复合物的信号对 NLRP2 和 NLRP3 的炎症小体传感器与 AIM2 的炎症小体传感器的早期不同调制。
阿尔茨海默病(AD)是人类最常见的痴呆症,其发病原因是细胞外 Aβ42 老年斑和细胞内高磷酸化 Tau(p-Tau)蛋白神经纤维缠结的累积。与先天性免疫相关的细胞膜蛋白寡聚体(被称为炎性体)会引发 AD 并发的神经炎症。在适当的 "第一"(启动)和 "第二"(激活)信号作用下,炎症小体过量产生促炎性白细胞介素(IL)-1β和IL-18,同时裂解促进发热的加斯明D的N端片段。我们早先的研究表明,在纯单培养基中,外源 Aβ25-35 处理过的非增殖人皮质星形胶质细胞(HCAs)会制造并释放过量的内源性 Aβ42-oligomers (-os) 和 p-Tau-os,就像同样处理过的人皮质神经元一样。经 Aβ25-35 暴露的 HCA 也会过度释放 NO、VEGFA 和 IL-6。Aβ-CaSR(Aβ-钙传感受体)复合物产生的细胞内信号介导了所有这些神经毒性效应,因为 CaSR 的负异位调节剂(又名 NAMs 或钙化剂,如 NPS2143)完全抑制了这些效应。然而,Aβ-CaSR 复合物发出的信号是否也会诱导单培养 HCA 中 NOD-like 2(NLRP2)和 3(NLRP3)以及黑色素瘤中缺失的PYHIN 2(AIM2)炎性体的早期表达和/或激活,这一点迄今仍未得到探讨。为了弄清这个问题,我们使用了原位邻近连接、qRT-PCR、双抗体阵列、免疫印迹和Caspase 1/4酶测定法。Aβ-CaSR复合物迅速在HCA表面聚集,并发出激活Akt和JAK/STAT轴的胞内信号。反过来,后者上调 NLRP2 和 NLRP3 PRRs(模式识别受体),同时下调 AIM2。这些效应具有特异性,NPS2143 和 PI3K(LY294002)、AMPKα(Dorsomorphin)、mTOR(Torin1)和 JAK/TYK (Brepoticinib)抑制剂都会明显阻碍这些效应。广谱抑制剂 Bay11-7082 强化了 Aβ-CaSR/Akt/JAK/STAT 轴对 NLRP3 和 AIM2 PRR 蛋白的相反控制,而不影响 NLRP2 PRR 的上调。然而,上述对 PRRs 蛋白的影响在 24 小时内消失。此外,Aβ-CaSR 信号既不会同时改变 ASC、pro-IL-1β、Gasdermin-D(全蛋白和片段)蛋白水平以及 Caspases 1 和 4 酶活性,也不会诱导细胞凋亡。因此,Aβ-CaSR 提示作为 "第一信号(启动信号)"暂时增加了 NLRP2 和 NLRP3 PRRs 的表达,但没有激活相应的炎症体。Aβ-CaSR 提示和 Bay11-7082 对 NLRP3 PRR 蛋白和 AIM2 PRR 蛋白的调控截然不同,这表明当细菌或病毒 DNA 片段缺失时,AIM2 可能在 HCAs 中发挥 "抗炎 "或其他作用。然而,Bay11-7082对NLRP2 PRR过表达没有影响,这也揭示了CaSR控制炎性体传感器的下游机制在HCAs中相当复杂,因此,鉴于AD对人类健康的影响,非常值得进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


