Photosensitizing metal–organic framework nanoparticles combined with tumor-sensitization strategies can enhance the phototherapeutic effect upon medullary thyroid carcinoma
IF 2.8
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. General subjects
Pub Date : 2024-10-19
DOI:10.1016/j.bbagen.2024.130725
引用次数: 0
Abstract
Photodynamic therapy (PDT) utilizing metal-organic frameworks (MOFs) has developed as a new and efficacious treatment for malignant tumors located on the surface of the human body. In order to achieve more effective PDT treatment outcomes, the traditional method has been to increase the intensity of the laser irradiation, but this approach can easily lead to tissue burns. In this study, we developed a new type of nanoparticle, F68-PKI@PCN224, aims to achieve effective PDT upon medullary thyroid carcinoma (MTC) which is an uncommon form of thyroid cancer that originates in the parafollicular cells of the thyroid and the therapeutic outlook for patients with MTC remains unsatisfactory. F68-PKI@PCN224 combines the antitumor features of PDT with mammalian target of rapamycin (mTOR) inhibitor PKI-587 (PKI). The tumor sensitization, slow release, and pH response features of F68-PKI@PCN224 was demonstrated by a series of in vitro and in vivo experiments / assays. F68-PKI@PCN224 achieved the long-term activation and slow releasing of PKI and TCPP in MTC tumor tissues. During the process of generating PDT effects, F68-PKI@PCN224 enhanced the tumor's sensitivity to PDT, direct laser irradiation of MTC cells or subcutaneous tumor tissues. As a result, low-dose phototherapy achieves a higher anti-tumor effect upon F68-PKI@PCN224 compared with TCPP. This study reveals the synergistic effect between tumor sensitization by mTOR inhibitor and PDT and initially unveils the mechanism of action of these nanoparticles.
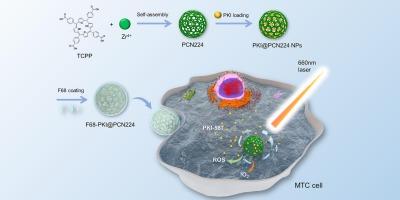
光敏金属有机框架纳米粒子与肿瘤增敏策略相结合,可增强对甲状腺髓样癌的光疗效果。
利用金属有机框架(MOFs)的光动力疗法(PDT)已发展成为一种治疗人体表面恶性肿瘤的新型有效疗法。为了实现更有效的 PDT 治疗效果,传统方法是增加激光照射强度,但这种方法很容易导致组织灼伤。甲状腺髓样癌是一种不常见的甲状腺癌,起源于甲状腺滤泡旁细胞,其治疗前景仍不令人满意。F68-PKI@PCN224将PDT的抗肿瘤特性与哺乳动物雷帕霉素靶点(mTOR)抑制剂PKI-587(PKI)相结合。一系列体内外实验证明了 F68-PKI@PCN224 的肿瘤敏化、缓释和 pH 响应特性。F68-PKI@PCN224 在 MTC 肿瘤组织中实现了 PKI 和 TCPP 的长期激活和缓释。在产生PDT效应的过程中,F68-PKI@PCN224增强了肿瘤对PDT、直接激光照射MTC细胞或皮下肿瘤组织的敏感性。因此,与 TCPP 相比,F68-PKI@PCN224 的低剂量光疗具有更高的抗肿瘤效果。这项研究揭示了 mTOR 抑制剂对肿瘤的增敏作用与光疗之间的协同效应,并初步揭示了这些纳米粒子的作用机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. General subjects
生物-生化与分子生物学
CiteScore
6.40
自引率
0.00%
发文量
139
审稿时长
30 days
期刊介绍:
BBA General Subjects accepts for submission either original, hypothesis-driven studies or reviews covering subjects in biochemistry and biophysics that are considered to have general interest for a wide audience. Manuscripts with interdisciplinary approaches are especially encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


