Cholic acid activation of GPBAR1 does not induce or exacerbate acute pancreatitis but promotes exocrine pancreatic secretion
IF 2.2
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-12
DOI:10.1016/j.bbrc.2024.150825
引用次数: 0
Abstract
Obstruction of bile ducts due to gallstones can lead to biliary acute pancreatitis (BAP). According to Perides et al., G protein-coupled bile acid receptor-1 (GPBAR1) mediates BAP. However, Zi's findings suggest that GPR39, rather than GPBAR1, mediates TLCAS-induced increases in cytosolic calcium and acinar cell necrosis, casting doubt on the role of GPBAR1 in BAP. Numerous G protein-coupled receptors on pancreatic acinar cells utilize Ca2+ and cyclic adenosine monophosphate (cAMP) as second messengers to manage pancreatic exocrine secretion, with significant cross-talk between these signals. The primary bile acid cholic acid (CA) and its conjugated forms are predominant in the human gallbladder. This study aimed to clarify the role and physiological significance of GPBAR1 by investigating the physiological and pathological effects of CA activation on GPBAR1 in pancreatic acinar cells. Isolated rat pancreatic acinar cells were treated with CA and CCK in vitro to observe the effect of CA-induced cAMP signaling on CCK-induced physiological and pathological calcium signaling. In vivo evaluations involved reverse biliopancreatic duct injections of 5 % sodium taurocholate (STC) or 5 % CA in rats. CA induced intracellular cAMP signaling in a concentration-dependent manner without increasing the intracellular Ca2+ concentration. CA did not independently cause calcium overload or enzyme activation, nor did it exacerbate calcium overload or enzyme activation from high-dose CCK. Reverse biliopancreatic duct injections of 5 % CA did not cause acute pancreatitis in the rats. Transcriptomic analysis revealed that 50 μM CA induced changes in gene expression related to protein synthesis in the endoplasmic reticulum and ribosomes. Furthermore, 50 μM CA accelerated the calcium waves and increased the enzyme secretion induced by CCK. GPBAR1 was found on the basolateral membrane in rat pancreatic tissue rather than near the apical region of acinar cells.
GPBAR1 activation is not crucial for BAP activity but may play a role in bile acid regulation of pancreatic exocrine secretion, suggesting that GPBAR1 is a potential therapeutic target for pancreatic exocrine insufficiency.
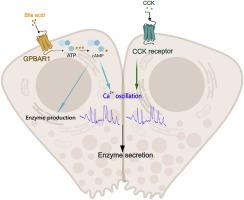
胆酸激活 GPBAR1 不会诱发或加重急性胰腺炎,但会促进胰腺外分泌。
胆结石阻塞胆管可导致胆源性急性胰腺炎(BAP)。根据 Perides 等人的研究,G 蛋白偶联胆汁酸受体-1(GPBAR1)介导胆源性急性胰腺炎。然而,Zi 的研究结果表明,GPR39,而不是 GPBAR1,介导了 TLCAS 诱导的细胞膜钙增加和尖塔细胞坏死,从而对 GPBAR1 在 BAP 中的作用产生了怀疑。胰腺针叶细胞上的许多 G 蛋白偶联受体利用 Ca2+ 和环磷酸腺苷(cAMP)作为第二信使来管理胰腺外分泌,这些信号之间存在显著的交叉作用。人体胆囊中主要存在胆汁酸胆酸(CA)及其共轭形式。本研究旨在通过研究 CA 激活对胰腺针叶细胞中 GPBAR1 的生理和病理影响,阐明 GPBAR1 的作用和生理意义。体外用 CA 和 CCK 处理分离的大鼠胰腺尖突细胞,观察 CA 诱导的 cAMP 信号转导对 CCK 诱导的生理和病理钙信号转导的影响。体内评估包括向大鼠胆胰管反向注射 5% 牛磺胆酸钠(STC)或 5% CA。CA 以浓度依赖性方式诱导细胞内 cAMP 信号传导,而不增加细胞内 Ca2+ 浓度。CA不会单独引起钙超载或酶活化,也不会加剧高剂量CCK引起的钙超载或酶活化。反向胆胰管注射 5 % CA 不会导致大鼠急性胰腺炎。转录组分析表明,50 μM CA 可诱导内质网和核糖体中与蛋白质合成有关的基因表达发生变化。此外,50 μM CA 还能加速钙波,增加 CCK 诱导的酶分泌。在大鼠胰腺组织中,GPBAR1 位于基底侧膜上,而不是尖细胞顶端附近。GPBAR1 的激活对 BAP 活性并不重要,但可能在胆汁酸调节胰腺外分泌中发挥作用,这表明 GPBAR1 是胰腺外分泌功能不全的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


