Impact of Lectin biotinylation for surface plasmon resonance and enzyme-linked Lectin assays for protein glycosylation
IF 2.6
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Lectins are widely employed for the assessment of protein glycosylation as their carbohydrate binding specificities have been well characterized. In glycosylation assays, lectins are often conjugated with biotin tags, which interact with streptavidin to functionalize biosensing surfaces or recruit signal generating molecules, depending on the assay configuration. We here demonstrate that a high degree of biotin conjugation can limit total capture to streptavidin functionalized SPR surfaces due to multipoint binding, and can additionally bias the reported kinetic evaluations when measuring the interaction between lectins and glycoproteins by SPR. For microplate assays using different configurations, high biotinylation ratios can effectively amplify the signal obtained when using Streptavidin conjugates for detection, in some cases significantly lowering the limit of detection. The cumulative results express the importance of customizing the ligand biotinylation ratios for different assay configurations, as commercially obtained pre-biotinylated lectins are not necessarily optimized for different assay configurations.
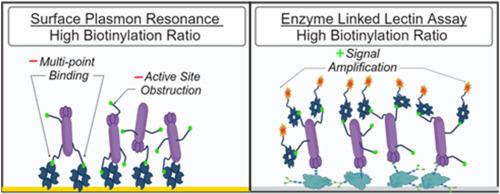
表面等离子共振和酶联凝集素检测蛋白质糖基化的凝集素生物素化的影响。
凝集素被广泛用于评估蛋白质糖基化,因为它们与碳水化合物结合的特异性已经得到了很好的表征。在糖基化检测中,凝集素通常与生物素标签连接,生物素标签与链霉亲和素相互作用,使生物传感表面功能化,或根据检测配置吸附信号生成分子。我们在此证明,由于多点结合,高度的生物素共轭会限制链霉亲和素功能化 SPR 表面的总捕获量,而且在通过 SPR 测量凝集素和糖蛋白之间的相互作用时,还会使报告的动力学评估出现偏差。对于使用不同配置的微孔板检测,当使用链霉亲和素共轭物进行检测时,高生物素化比率可有效放大获得的信号,在某些情况下可显著降低检测限。累积的结果表明了针对不同的检测配置定制配体生物素化比率的重要性,因为市售的预生物素化凝集素并不一定针对不同的检测配置进行了优化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


