Dermal toxicity studies of 1,2-benzisothiazolin-3-one (CAS number: 2634-33-5) in Sprague-Dawley rats
IF 3.9
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Biocides are used as preservatives in various household products, and 1,2-benzisothiazolin-3-one (BIT) is one of the popular chemicals. Therefore, BIT is highly likely to be exposed to human skin, necessitating dermal toxicity evaluation. In this study, we aimed to investigate dermal toxicity, eyes and skin irritation, and skin sensitization of BIT. All studies were conducted according to the Organisation for Economic Co-operation and Development testing guidelines. In acute dermal toxicity using rats, no treatment-related responses were observed at the highest dose (2000 mg/kg). A 28-day repeated dermal toxicity study at 1, 4, and 12 mg/kg/day showed transient local skin irritation lesions, including erythema, exfoliation, and crust formation. Based on no systemic effects, the no observed adverse effect level (NOAEL) of BIT of the 28-day repeated dermal toxicity study was determined to be 12 mg/kg/day. Eye and skin irritation tests showed that BIT is a strong irritant and corrosive to the eyes and a mild irritant to the skin. However, BIT showed no skin sensitization reactions in a local lymph node assay. These dermal toxicity studies can provide valuable information for the risk assessment of BIT.
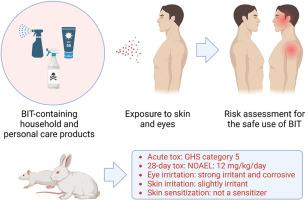
1,2-苯并异噻唑啉-3-酮(化学文摘社编号:2634-33-5)对 Sprague-Dawley 大鼠的皮肤毒性研究。
杀菌剂被用作各种家用产品的防腐剂,1,2-苯并异噻唑啉-3-酮(BIT)是其中一种常用化学品。因此,BIT 极有可能接触到人体皮肤,因此有必要进行皮肤毒性评估。本研究旨在调查 BIT 的皮肤毒性、眼睛和皮肤刺激性以及皮肤致敏性。所有研究都是根据经济合作与发展组织的测试指南进行的。在使用大鼠进行的急性皮肤毒性试验中,最高剂量(2000 毫克/千克)未观察到与治疗相关的反应。以 1、4 和 12 毫克/千克/天的剂量进行的为期 28 天的重复皮肤毒性研究显示,出现了短暂的局部皮肤刺激症状,包括红斑、脱皮和结痂。由于未出现系统性影响,28 天重复皮肤毒性研究中 BIT 的无观测不良效应水平 (NOAEL) 被确定为 12 毫克/千克/天。眼睛和皮肤刺激测试表明,BIT 对眼睛有强烈的刺激性和腐蚀性,对皮肤有轻微的刺激性。不过,在局部淋巴结试验中,BIT 未显示皮肤过敏反应。这些皮肤毒性研究可为 BIT 的风险评估提供有价值的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


