Engineering cell membrane-camouflaged COF-based nanosatellite for enhanced tumor-targeted photothermal chemoimmunotherapy
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Dendritic cells (DCs) activation is crucial for regulating the antitumor immune response. However, the tumor's immunosuppressive environment significantly impedes antigen presentation and DCs maturation, thereby limiting the effectiveness of cancer immunotherapy. To address this challenge, we developed tumor cell membrane-coated covalent organic framework (COF) nanoparticles, loaded with mannose-modified gold nanoparticles and doxorubicin (Dox). This created a cell membrane-camouflaged COF-based nanosatellite designed to enhance tumor-targeted chemoimmunotherapy. The nanosatellite exhibits distinct photothermal properties and releases Dox in a pH-sensitive manner, targeting tumor cells to induce immunogenic cell death (ICD) and expose a wealth of antigens. Crucially, the COF structure is selectively degraded to release mannose-modified gold nanoparticles in the acidic environment. These nanoparticles capture antigens from the ICD and efficiently transport them to lymph nodes rich in DCs, facilitated by mannose receptor mediation. As a result, antigens are effectively presented to DCs, activating the immune response, significantly hindering tumor growth and lung metastasis in mice, and extending survival. This study pioneered innovative nano-preparations aimed at enhancing tumor immunotherapy.
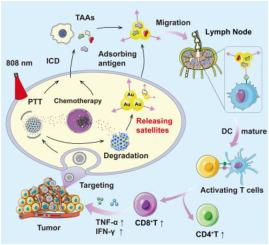
基于细胞膜伪装的 COF 纳米卫星工程,用于增强肿瘤靶向光热化疗免疫疗法。
树突状细胞(DCs)的激活对于调节抗肿瘤免疫反应至关重要。然而,肿瘤的免疫抑制环境极大地阻碍了抗原呈递和树突状细胞的成熟,从而限制了癌症免疫疗法的效果。为了应对这一挑战,我们开发了肿瘤细胞膜包被共价有机框架(COF)纳米粒子,其中装载了甘露糖修饰的金纳米粒子和多柔比星(Dox)。这样就产生了一种细胞膜掩蔽的基于 COF 的纳米卫星,旨在增强肿瘤靶向化疗免疫疗法。这种纳米卫星具有独特的光热特性,能以对 pH 值敏感的方式释放 Dox,靶向肿瘤细胞诱导免疫原性细胞死亡(ICD),并暴露大量抗原。最重要的是,COF 结构在酸性环境中会被选择性降解,释放出甘露糖修饰的金纳米粒子。这些纳米粒子能捕获 ICD 中的抗原,并在甘露糖受体介导下将其有效地输送到富含 DC 的淋巴结。因此,抗原能有效地呈现给直流电,激活免疫反应,显著阻碍小鼠的肿瘤生长和肺转移,延长生存期。这项研究开创了旨在增强肿瘤免疫疗法的创新纳米制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


