Anti-pyroptosis biomimetic nanoplatform loading puerarin for myocardial infarction repair: From drug discovery to drug delivery
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Pyroptosis is a critical pathological mechanism implicated in myocardial damage following myocardial infarction (MI), and the crosstalk between macrophages and pyroptotic cardiomyocytes presents a formidable challenge for anti-pyroptosis therapies of MI. However, as single-target pyroptosis inhibitors frequently fail to address this crosstalk, the efficacy of anti-pyroptosis treatment post-MI remains inadequate. Therefore, the exploration of more potent anti-pyroptosis approaches is imperative for improving outcomes in MI treatment, particularly in addressing the crosstalk between macrophages and pyroptotic cardiomyocytes. Here, in response to this crosstalk, we engineered an anti-pyroptosis biomimetic nanoplatform (NM@PDA@PU), employing polydopamine (PDA) nanoparticles enveloped with neutrophil membrane (NM) for targeted delivery of puerarin (PU). Notably, network pharmacology is deployed to discern the most efficacious anti-pyroptosis drug (puerarin) among the 7 primary active monomers of TCM formulations widely applied in clinical practice and reveal the effect of puerarin on the crosstalk. Additionally, targeted delivery of puerarin could disrupt the malignant crosstalk between macrophages and pyroptotic cardiomyocytes, and enhance the effect of anti-pyroptosis by not only directly inhibiting cardiomyocytes pyroptosis through NLRP3-CASP1-IL-1β/IL-18 signal pathway, but reshaping the inflammatory microenvironment by reprogramming macrophages to anti-inflammatory M2 subtype. Overall, NM@PDA@PU could enhance anti-pyroptosis effect by disrupting the crosstalk between M1 macrophages and pyroptotic cardiomyocytes to protect cardiomyocytes, ameliorate cardiac function and improve ventricular remodeling, which providing new insights for the efficient treatment of MI.
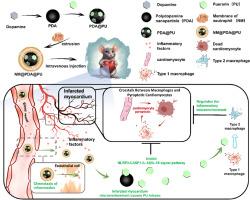
用于心肌梗死修复的装载葛根素的抗血栓形成生物仿生纳米平台:从药物发现到药物输送。
心肌梗塞(MI)后心肌损伤的一个重要病理机制是嗜热症,巨噬细胞和嗜热心肌细胞之间的串扰给心肌梗塞的抗嗜热症疗法带来了巨大挑战。然而,由于单一靶点的嗜热抑制剂往往无法解决这种串扰,因此心肌梗死后抗嗜热治疗的疗效仍然不足。因此,为了改善心肌梗死治疗的效果,探索更有效的抗嗜热细胞增多方法势在必行,尤其是在解决巨噬细胞和嗜热心肌细胞之间的串扰方面。在这里,针对这种串扰,我们设计了一种抗嗜脓细胞增多的仿生物纳米平台(NM@PDA@PU),利用包裹着中性粒细胞膜(NM)的多巴胺(PDA)纳米颗粒靶向递送葛根素(PU)。值得注意的是,该研究利用网络药理学从广泛应用于临床的中药制剂的 7 种主要活性单体中找出了最有效的抗肺脓肿药物(葛根素),并揭示了葛根素对串联效应的影响。此外,葛根素的靶向给药不仅能通过NLRP3-CASP1-IL-1β/IL-18信号通路直接抑制心肌细胞的脓毒症,还能通过将巨噬细胞重编程为抗炎的M2亚型来重塑炎症微环境,从而破坏巨噬细胞与脓毒症心肌细胞之间的恶性串联,增强抗脓毒症的效果。总之,NM@PDA@PU可通过破坏M1巨噬细胞和脓毒性心肌细胞之间的串联,增强抗脓毒症的效果,从而保护心肌细胞、改善心脏功能和心室重塑,为心肌梗死的高效治疗提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


