C3-Chlorination of C2-substituted benzo[b]thiophene derivatives in the presence of sodium hypochlorite†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Benzo[b]thiophene rings are common synthons for the development of novel drugs and materials, and thus, the discovery of facile ways for their functionalization is of value. In this work, a new method for the C3-chlorination of C2-substituted benzothiophene derivatives is described. The chlorine source is sodium hypochlorite pentahydrate (NaOCl·5H2O), and optimal transformations occur in aqueous acetonitrile at 65–75 °C to provide the corresponding C3-halogenated products in variable yields (30–65%). The reaction occurs in the presence of vinyl and alkyl groups, while the presence of alcohols leads to competing oxidation reactions at the heterobenzylic position. The presence of a carbonyl group at the C2-position inhibited the halogenation reaction, while the use of benzofuran led to a highly exothermic reaction, presumably via the formation of a peroxide intermediate. Reactions carried out at lower temperatures led to side reactions associated with competing oxidative processes. To gain a better understanding of the mechanism of the reaction, DFT calculations were carried out, where the heteroatom enables the formation of a hypochlorous acidium ion that serves to generate a C2–C3 chloronium ion intermediate in a step-wise manner, which in turn leads to the formation of an S-stabilized C2-carbocation that undergoes re-aromatization to the corresponding C3-chlorinated products. To probe potential synthetic applications, a model C3-chloro derivative was coupled with phenylboronic acid using standard Suzuki–Miyaura coupling conditions.
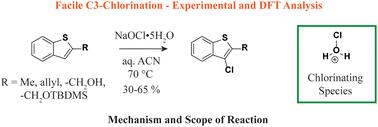
C2 取代的苯并[b]噻吩衍生物在次氯酸钠存在下的 C3 氯化反应。
苯并[b]噻吩环是开发新型药物和材料的常见合成物,因此,发现其功能化的简便方法具有重要价值。本文介绍了一种 C2 取代苯并噻吩衍生物的 C3 氯化新方法。氯源为五水次氯酸钠(NaOCl-5H2O),在 65-75 °C 的乙腈水溶液中进行最佳转化,可提供相应的 C3 卤化产物,产率可变(30-65%)。反应在乙烯基和烷基存在的情况下发生,而醇的存在会导致杂苄基位置发生竞争性氧化反应。C2 位上羰基的存在抑制了卤化反应,而苯并呋喃的使用导致了高放热反应,可能是通过形成过氧化物中间体。在较低温度下进行的反应会导致与竞争氧化过程相关的副反应。为了更好地了解反应机理,我们进行了 DFT 计算,其中杂原子使次氯酸根离子得以形成,次氯酸根离子以分步的方式生成 C2-C3 氯离子中间体,进而形成 S 稳定的 C2-配位体,该配位体经过再芳香化作用生成相应的 C3-氯化产物。为了探究潜在的合成应用,我们采用标准的铃木-宫浦偶联条件将模型 C3-氯衍生物与苯硼酸偶联。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


