Mechanistic insight into hydroxy-methylation of hardwood Kraft lignin
Abstract
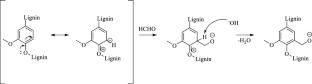
In view of developing upcycling strategies for hardwood Kraft lignin, hydroxy-methylation of Eucalyptus Kraft lignin under alkaline conditions (pH 9 and 11) at different temperatures (50 °C and 70 °C) was studied in the present effort with the double objective of optimizing the reaction conditions and understanding the functionalization mechanism of C5 in either terminal or internal guaiacyl units during hydroxy-methylation. Formaldehyde consumption was estimated via titration of the oximated free formaldehyde; the hydroxy-methylation degree under the reaction was estimated by calculating the ratio in Condensed hydroxyl/Guaiacyl (Condensed OH/G-OH) via a new difference UV-spectroscopy. The reliability of the difference UV-method results for the analyses of the hydroxy-methylated lignins was statistically analysed and compared with that of vacuum-dried and sonicated samples. Hydroxy-methylated samples were then fully characterised by NMR (31P and HSQC) and GPC. The reaction temperature of 50 °C, pH 11, and period time of one hour resulted as the optimal conditions for the hydroxy-methylation, preventing the side-reactions leading to the formation of dimethylene-glycol addition products. The 31P and 1H–13C HSQC NMR revealed the absence of undesirable formaldehyde Cannizzaro by-products and the lack of hydroxymethyl groups in the aliphatic side chain under the studied conditions. GPC analyses, comparing two methodologies, revealed increases in molar mass of the hydroxy-methylated samples upon the formaldehyde addition. The selective hydroxy-methylation at the C5 guaiacyl site demonstrates that Eucalyptus Kraft lignin is as a promising candidate for resol production.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


