Anion intercalation of NiMn-LDH accelerating urea electrooxidation on trivalent nickel
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
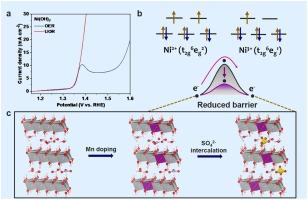
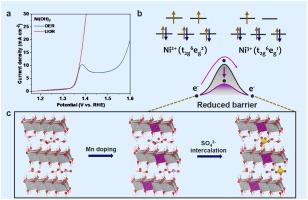
Reducing the urea oxidation reaction (UOR) barriers is a key knot for accelerating its practical applications. Here, we demonstrate that NiMn-LDH with sulfate anion interaction can enable urea electrooxidation with a low anodic potential of 1.36 V at 100 mA cm−2. We find that the UOR on NiMn-LDH is driven by Ni3+ species and the Ni3+ generation is the rate-determining step of UOR. Both the Mn doping and sulfate anion interaction contribute to the low-barrier phase transformation from Ni(OH)2 to NiOOH to produce the Ni3+ state with high activity in UOR, owing to that Mn doping optimizes the electronic states and intercalation of guest anions weakens the interlayer interactions, which ultimately tunes Ni3+ generation kinetics toward the superior UOR activity. Our findings provide new insights into the design of the highly active UOR catalysts.
NiMn-LDH 的阴离子插层加速三价镍上的尿素电氧化作用
降低尿素氧化反应(UOR)的障碍是加速其实际应用的关键。在此,我们证明了与硫酸根阴离子相互作用的 NiMn-LDH 可在 100 mA cm-2 条件下以 1.36 V 的低阳极电位实现尿素电氧化。我们发现,NiMn-LDH 上的尿素电氧化是由 Ni3+ 物种驱动的,Ni3+ 的生成是尿素电氧化的决定性步骤。掺杂锰和硫酸根阴离子的相互作用都有助于从 Ni(OH)2 到 NiOOH 的低势垒相变,从而在 UOR 中产生具有高活性的 Ni3+ 态,这是因为掺杂锰优化了电子态,而客体阴离子的插层削弱了层间相互作用,最终调整了 Ni3+ 生成动力学,使其具有更高的 UOR 活性。我们的发现为高活性 UOR 催化剂的设计提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


