Photoredox-catalysed amidyl radical insertion to bicyclo[1.1.0]butanes
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Replacing planar aromatic rings in drug molecules with C(sp3)-rich isosteric mimetics, such as bicyclo[n.1.1]alkanes, can significantly alter their physicochemical and pharmacokinetic properties, often leading to higher clinical success rates. However, unlike a benzene ring, the structurally rigid C(sp3)-rich isosteric mimetics of heteroaromatic rings are rare. Heterobicyclo[n.1.1]alkanes are promising in this regard, but the lack of modular synthetic methods has currently hindered their exploration. We envisioned that the strategic and selective insertion of different heteroatomic units to bicyclo[1.1.0]butanes could offer a highly modular platform to access diverse heterobicyclo[n.1.1]alkanes. Herein we report a photoredox-catalysed highly regioselective and chemoselective insertion of amidyl radicals to bicyclo[1.1.0]butanes, providing direct access to 2-oxa-4-azabicyclo[3.1.1]hept-3-enes. The exit vector analysis shows a geometric resemblance of these C(sp3)-rich heterobicyclic motifs with pyridine and pyrimidine derivatives, suggesting their potential as isosteric mimetics of such medicinally important heterocycles. Additionally, various downstream transformations demonstrate their utility as versatile building blocks in synthetic chemistry. Heteroatom-substituted C(sp3)-rich polycyclic hydrocarbon rings, isosteric to heterocyclic rings, are not common due to the challenging synthesis. Now a photoredox-catalysed strategy to insert amidyl radicals into bicyclo[1.1.0]butanes is presented, providing direct access to 2-oxa-4-azabicyclo[3.1.1]hept-3-enes.
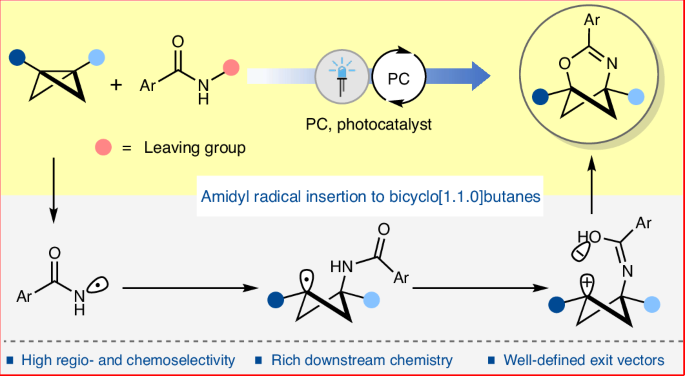
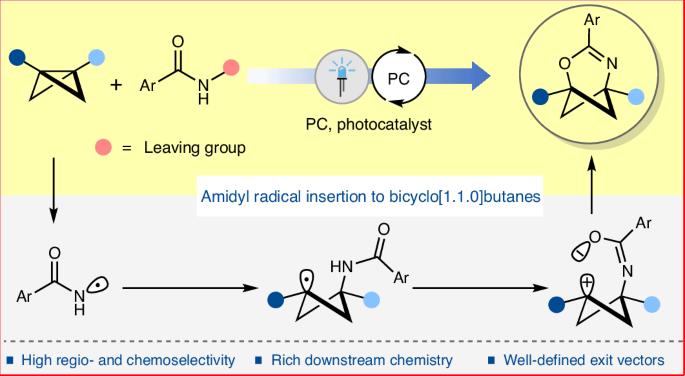
光氧化催化酰胺基插入双环[1.1.0]丁烷
用双环[n.1.1]烷烃等富含 C(sp3)的异构模拟物取代药物分子中的平面芳香环,可以显著改变药物分子的理化和药代动力学特性,往往能提高临床成功率。然而,与苯环不同的是,杂芳香环的结构刚性C(sp3)丰富的异构模拟物非常罕见。杂双环[n.1.1]烷烃在这方面大有可为,但目前缺乏模块化合成方法阻碍了对它们的探索。我们设想,将不同的杂原子单元策略性地、选择性地插入双环[1.1.0]丁烷,可以提供一个高度模块化的平台,以获得多种杂双环[n.1.1]烷烃。在此,我们报告了一种光氧化催化的高区域选择性和化学选择性酰胺基插入双环[1.1.0]丁烷的方法,它提供了直接获得 2-oxa-4-azabicyclo[3.1.1]hept-3-enes 的途径。出口矢量分析表明,这些富含 C(sp3)的杂双环基团与吡啶和嘧啶衍生物具有几何相似性,这表明它们有可能成为这些具有重要药用价值的杂环的同位模拟物。此外,各种下游转化过程也证明了它们在合成化学中作为多功能构建模块的用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


