Integrated non-targeted metabolomics and lipidomics reveal mechanisms of fluorotelomer sulfonates-induced toxicity in human hepatocytes
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
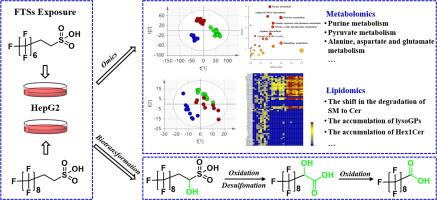
Fluorotelomer sulfonates (FTSs) are widely used as novel substitutes for perfluorooctane sulfonate, inevitably leading to FTSs accumulation in various environmental media and subsequent exposure to humans. This accumulation eventually poses environmental hazards and health risks. However, their toxicity mechanisms remain unclear. Herein, the mechanisms of two FTSs (6:2 and 8:2 FTS) induced toxicity in human hepatocellular carcinoma cells were investigated via non-targeted metabolomics and lipidomics based on liquid chromatography–high resolution mass spectrometry. Our results revealed that amino acid, purine, acylcarnitine and lipid levels were significantly perturbed by 6:2 and 8:2 FTS exposure. The effects of 8:2 FTS exposure were largely characterized by up-regulation of pyruvate metabolism pathway and down-regulation of purine metabolism pathway, whereas the opposite trends were induced by 6:2 FTS exposure. The opposite trends were confirmed by the mRNA expression levels of four key genes (glyoxalase 1, adenylosuccinate lyase, inosine monophosphate dehydrogenase 1 (IMPDH1) and IMPDH2) determined by real-time PCR. Common lipid perturbations included significantly increased ceramide/sphingomyelin ratios, and obvious accumulation of hexosylceramides and lysoglycerophospholipids. 6:2 FTS exposure induced sharp accumulation of glycerides, including monoglycerides, diglycerides and triglycerides. 8:2 FTS exposure induced decreased levels of acylcarnitines and fatty acids. Both of 6:2 and 8:2 FTS exposure induced increased levels of intracellular reactive oxygen species, an imbalance in energy metabolism homeostasis, and mitochondrial dysfunction. The results of integrated omics analysis are expected to serve as valuable information for the health risk assessment of 6:2 FTS and 8:2 FTS.
综合非靶向代谢组学和脂质组学揭示氟特罗姆磺酸盐诱导人肝细胞毒性的机制
氟橡胶磺酸盐(FTSs)作为全氟辛烷磺酸盐的新型替代品被广泛使用,不可避免地会导致 FTSs 在各种环境介质中积累,进而导致人体接触。这种积累最终会造成环境危害和健康风险。然而,它们的毒性机制仍不清楚。本文基于液相色谱-高分辨质谱法,通过非靶向代谢组学和脂质组学研究了两种 FTS(6:2 和 8:2 FTS)诱导人肝癌细胞毒性的机制。结果表明,6:2 和 8:2 FTS 暴露对氨基酸、嘌呤、酰基肉碱和脂质水平产生了显著干扰。暴露于 8:2 FTS 的影响主要表现为丙酮酸代谢途径的上调和嘌呤代谢途径的下调,而暴露于 6:2 FTS 则表现出相反的趋势。通过实时 PCR 检测四个关键基因(乙醛酸酶 1、腺苷琥珀酸裂解酶、单磷酸肌苷脱氢酶 1 (IMPDH1) 和 IMPDH2)的 mRNA 表达水平,证实了这种相反的趋势。常见的脂质扰动包括神经酰胺/鞘磷脂比率明显增加,以及己糖基甘油酯和溶血甘油磷脂的明显积累。接触 6:2 FTS 会导致甘油酯(包括单甘油酯、双甘油酯和甘油三酯)急剧积累。接触 8:2 FTS 会导致酰基肉碱和脂肪酸水平下降。暴露于 6:2 和 8:2 FTS 会导致细胞内活性氧水平升高、能量代谢平衡失调和线粒体功能障碍。综合全息分析的结果有望为 6:2 FTS 和 8:2 FTS 的健康风险评估提供有价值的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


