Potent efficacy of an IgG-specific endoglycosidase against IgG-mediated pathologies
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Endo-β-N-acetylglucosaminidases (ENGases) that specifically hydrolyze the Asn297-linked glycan on immunoglobulin G (IgG) antibodies, the major molecular determinant of fragment crystallizable (Fc) γ receptor (FcγR) binding, are exceedingly rare. All previously characterized IgG-specific ENGases are multi-domain proteins secreted as an immune evasion strategy by Streptococcus pyogenes strains. Here, using in silico analysis and mass spectrometry techniques, we identified a family of single-domain ENGases secreted by pathogenic corynebacterial species that exhibit strict specificity for IgG antibodies. By X-ray crystallographic and surface plasmon resonance analyses, we found that the most catalytically efficient IgG-specific ENGase family member recognizes both protein and glycan components of IgG. Employing in vivo models, we demonstrated the remarkable efficacy of this IgG-specific ENGase in mitigating numerous pathologies that rely on FcγR-mediated effector functions, including T and B lymphocyte depletion, autoimmune hemolytic anemia, and antibody-dependent enhancement of dengue disease, revealing its potential for treating and/or preventing a wide range of IgG-mediated diseases in humans.
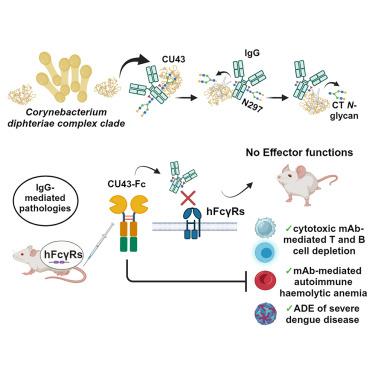
IgG特异性内糖苷酶对IgG介导的病症具有强大疗效
内切-β-N-乙酰葡糖胺酶(ENGases)能特异性水解免疫球蛋白 G(IgG)抗体上与 Asn297 连接的聚糖(IgG 是片段结晶(Fc)γ 受体(FcγR)结合的主要分子决定因素),但这种酶极为罕见。所有先前表征的 IgG 特异性恩格酶都是化脓性链球菌菌株作为一种免疫逃避策略分泌的多域蛋白。在这里,我们利用硅学分析和质谱技术,确定了致病性球孢子菌分泌的单链ENG酶家族,它们对IgG抗体具有严格的特异性。通过 X 射线晶体学和表面等离子体共振分析,我们发现催化效率最高的 IgG 特异性ENGase 家族成员既能识别 IgG 的蛋白质成分,也能识别 IgG 的聚糖成分。利用体内模型,我们证明了这种 IgG 特异性ENGase 在减轻依赖 FcγR 介导的效应器功能的多种病症(包括 T 淋巴细胞和 B 淋巴细胞耗竭、自身免疫性溶血性贫血和抗体依赖性登革热病增强)方面的显著功效,揭示了它在治疗和/或预防人类多种 IgG 介导的疾病方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


